and vessel cooption. The tumor microenvironment including hypoxia, glucose deprivation, and low pH plays an important role in the initiation of tumor angiogenesis via the activation of oncogenes and/or inactivation of tumor suppressor genes.3
 Figure 32-1. Distribution of contrast agent within the vascular bed of the organ of interest after intravenous administration. |
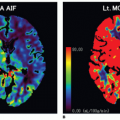
However, one of the challenges of clinicopathologic correlative studies is the comparison of in vivo with ex vivo findings. Tacelli et al. have showed that the areas of low regional tumor blood volume but high permeability have higher CD34 expression (assessed in three hotspots in the nonnecrotic tumor portion) than areas of high regional tumor blood volume and high permeability: 72.1 versus 47.9, p = 0.038.17 They postulated this difference could be related to the effect of the tumor microenvironment.17
TABLE 32.1 Definition of Vascular Parameters | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
 Figure 32-4. Schema showing typical perfusion CT acquisition for tumor evaluation and suggested contrast agent injection rate. |
For the initial perfusion phase, an acquisition duration of 45 seconds is adequate; for the interstitial phase at least five additional time points are recommended, the sampling rate ranging from 5 to 15 seconds depending on the kinetic model applied.1 As the concentration of iodine within blood vessels and tissues is proportional to the resultant increase in attenuation, temporal changes in attenuation can be analyzed using standard kinetic models without prior conversion to iodine concentration.
TABLE 32.2 Summary of the Commonly Applied Kinetic Models | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
may be seen depending on the mechanism of action of the drug under investigation and timing of the scan. An initial effect may be a decrease in vascular permeability and reduction in interstitial fluid pressure, with normalization of function of the vasculature resulting in a transient increase in tumor blood flow.53 In the longer term, with subsequent pruning of the vasculature, a reduction in regional blood flow, blood volume, and vascular permeability may be elicited (Fig. 32-6). With vascular disrupting agents, which target the proliferating immature vasculature +/- the mature vasculature a rapid shutdown in tumor vascularization may occur that is usually transient and reversible within 24 to 48 hours. This may be followed by a rebound revascularization.54 With radiotherapy, the acute effects are related to an initial inflammatory effect; the permeability is related to microvascular damage, which can lead to tumor shrinkage.55 With interventional procedures perfusion CT parameters may provide evidence of effective treatment or the need for further procedural attempts for optimal therapeutic effect.56
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree