At ultrasound (Fig. 10.2), ascites is seen as echo-free regions with excellent transmission of sound. Ultrasound may be used to direct the insertion of a pigtail catheter into ascites in order to drain the fluid. When loculated, ascites is seen as discrete collections of fluid partly surrounded by bands of adhesions and partly by the edges of normal abdominal structures, e.g. bowel wall or liver edge. Loculated collections of ascites may not be distinguishable from fluid in abscesses because the density of infected and uninfected fluid can be identical.
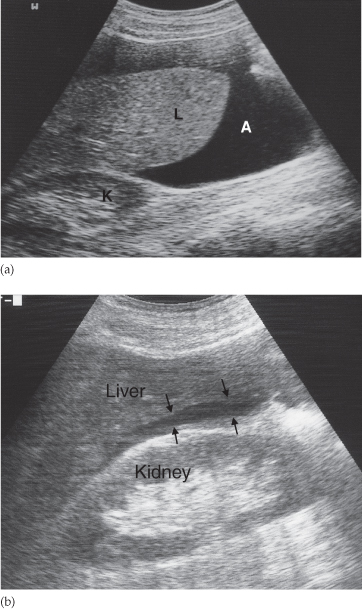
Fig. 10.2 Ultrasound of ascites. (a) The ascites (A) appears as a transonic area. The liver is clearly seen surrounded by the ascitic fluid. K, right kidney; L, liver. (b) Only a very small amount of ascites is present in this patient. The ascites (arrows) lies between the liver and kidney in Morrison’s pouch.
Pseudomyxoma peritoneii is a type of loculated intraperitoneal fluid of high viscosity, associated with mucinous tumours that have disseminated in the peritoneal cavity.
Plain film detection of ascites is discussed in Chapter 5.
Peritoneal Tumours
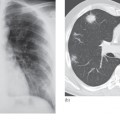
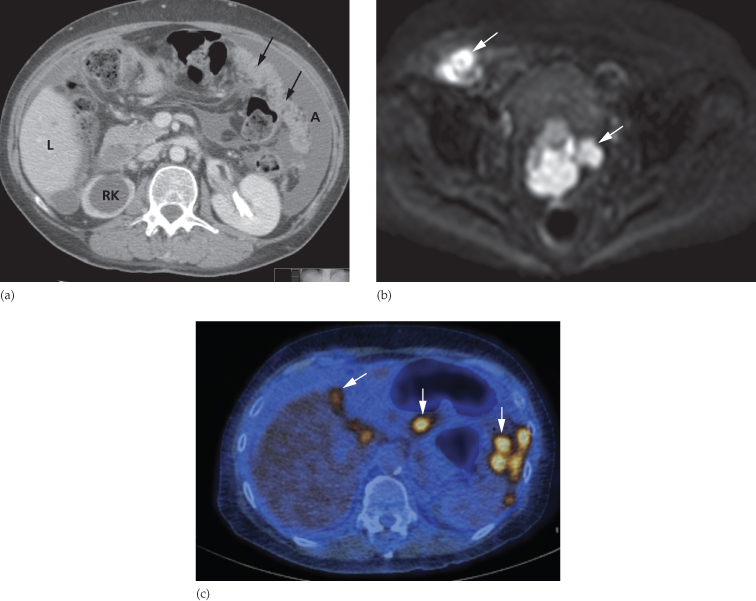
By far the most frequent neoplastic disease of the peritoneum is metastases from an abdominal or pelvic tumour, particularly carcinoma of the ovary. The peritoneum may be studded with tiny nodules that are frequently too small to detect on imaging and, in these cases, ultrasound and CT may only show ascites. In other cases, peritoneal nodules are clearly demonstrated on CT. Numerous metastatic nodules within the omental fat may coalesce to form what is known as an ‘omental cake’ (Fig. 10.3a). Peritoneal deposits may be seen on MRI, particularly using the diffusion-weighted sequence, in which deposits are typically of high signal intensity on the high b value images (Fig. 10.3b). Fluorodeoxyglucose positron emission tomography (FDG-PET)/CT may also be used to demonstrate the extent of peritoneal disease in tumours that are highly metabolically active (Fig. 10.3c). Ultrasound or CT-guided biopsy of the omental cake may be performed in cases where a tissue diagnosis is required prior to treatment planning, particularly as several pathologies may mimic ovarian cancer (Box 10.1). Carcinoid tumours of the small bowel may metastasize to form a mesenteric mass (Fig. 10.4). These are often partially calcified and may cause a desmoplastic reaction in the surrounding mesentery.
Fig. 10.3 Intraperitoneal spread of ovarian carcinoma in three different patients. (a) CT demonstrating ascites (A) and an ‘omental cake’ of tumour deposits (arrows). There is hydronephrosis of the right kidney (RK). L, liver. (b) Diffusion-weighted MRI, b 1000 image, demonstrating the high signal intensity of peritoneal deposits (arrows). (c) Fused FDG-PET/CT demonstrating multiple FDG-avid peritoneal deposits (arrows) in the upper abdomen.
- Primary peritoneal malignancy
- Disseminated gastrointestinal malignancy (e.g. stomach or colon)
- Disseminated pancreatic cancer
- Disseminated breast cancer (usually lobular subtype)
- Tuberculosis
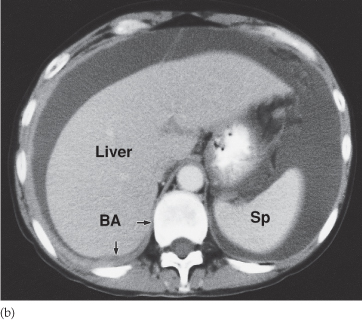
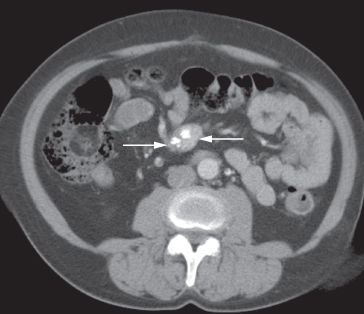
Fig. 10.4 CT demonstrating a mass in the small bowel mesentery (arrows), which contains several dense calcifications. This is a secondary deposit from an ileal carcinoid tumour.
Intraperitoneal Abscesses
Intraperitoneal abscesses may follow a perforation of the bowel or biliary tract, either spontaneous or post-traumatic, particularly following surgery. In the case of bowel perforation, free intraperitoneal air may be dectected in addition to the abscess. The common locations are subphrenic, subhepatic, paracolic and pelvic. The expected site of the abscess depends on the site of perforation, e.g. duodenal leakage usually results in subhepatic or subphrenic abscesses, whereas a leak from the left colon often results in paracolic or pelvic abscesses. Multiple abscesses are not uncommon following a perforation. Ascites frequently accompanies abdominal and pelvic abscesses.
Computed tomography demonstrates most abscesses and rarely gives false-positive results. Ultrasound can be just as informative, provided it is positive, and may be preferred because it is quick and simple to perform, and may be done at the bedside in patients on intensive care. The disadvantage of ultrasound is that gas in dilated loops of bowel can interfere with the images and, therefore, abscesses may be overlooked.
Ultrasound Appearances
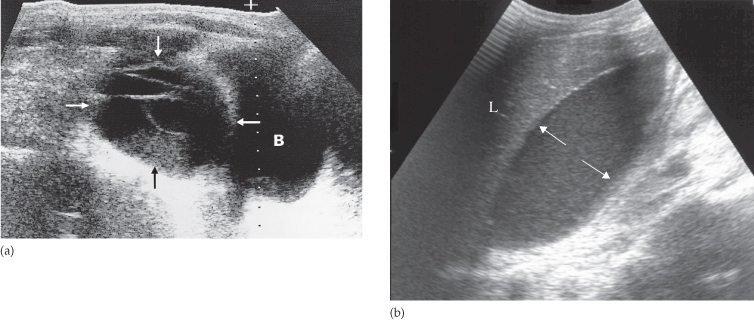
The ultrasonographer searches for any localized fluid collections lying outside the bowel. Abscesses assume many different configurations depending on the adjacent organs (Fig. 10.5). They often have slightly irregular walls and may contain internal echoes due to septations or debris. These internal echoes are not, however, specific for infection. Gas in an abscess appears echogenic and produces acoustic shadowing but it can be difficult to distinguish from gas within the bowel.
Fig. 10.5 Intraperitoneal abscess shown by ultrasound in two separate patients. (a) A large complex mass (arrows) just above the bladder (B). This young man had Crohn’s disease. (b) A large abscess (arrows) lying directly beneath the liver (L).
The major differential diagnosis is from loops of bowel and uninfected loculations of ascites, blood or lymph, all of which can occur postoperatively. The distinction may require needle aspiration of the fluid.
Computed Tomography Appearances
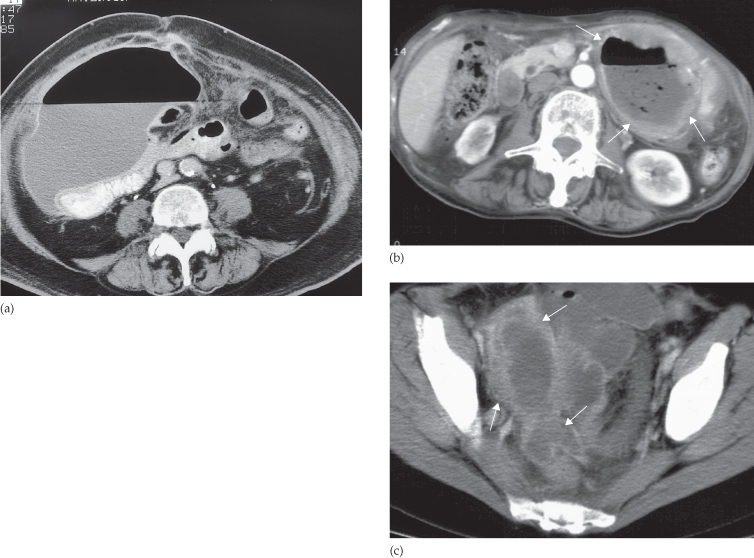
The basic principles are similar to those described for ultrasound. At CT, the fluid centre of the abscess is identified as a homogeneous density surrounded by a definite wall of soft tissue lying within the peritoneal cavity but outside the bowel (Fig. 10.6). Gas within the abscess is seen in approximately half the patients and is a very useful sign because it helps distinguish infected from uninfected fluid loculations. The gas may take the form of multiple small streaks or bubbles, or it may collect as one large bubble. Air–fluid levels may be present within larger collections. The wall of the abscess often shows enhancement following intravenous contrast administration. Subphrenic abscesses may be difficult to distinguish from pleural empyemas at CT; the peritoneal and pleural cavities are, after all, separated only by the diaphragm, a structure that can be difficult to identify at CT. In such cases, coronal or sagittal reformat of the CT or ultrasound will usually be very helpful because it can demonstrate the position of the abscess in relation to the diaphragm.
Fig. 10.6 CT of postoperative intraperitoneal abscesses in three different patients. (a) Large abscess in the right side of the abdomen at the level of the umbilicus. Note the large air–fluid collection with a thin enhancing wall. The abscess displaces adjacent bowel loops (containing air and oral contrast). (b) A typical thick-walled abscess (arrows) containing both air and fluid. (c) A pelvic abscess (arrows) containing fluid (pus).
The major diagnostic problems are:
- Distinguishing between abscesses and distended or matted loops of bowel. The distinction at CT can usually be made by opacifying the bowel. With ultrasound, peristalsis of bowel loops can be observed directly.
- Distinguishing between infected and uninfected fluid loculations; needle aspiration may have to be performed.
Magnetic Resonance Imaging Appearances
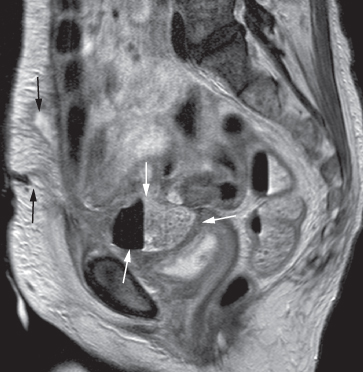
Pelvic abscesses are occasionally assessed on MRI, and usually have the appearance of a complex septate cyst with a thick enhancing wall. MRI may be helpful in delineating fistulous connections between the abscess and other organs, such as the bladder, bowel or vagina (Fig. 10.7).
Fig. 10.7 T2-weighted MRI scan in the sagittal plane demonstrating a pelvic abscess (white arrows) containing faecal material and air, which has formed a fistula with the anterior abdominal wall (black arrows).
Radionuclide Examination
If CT and ultrasound have been unsuccessful in locating an abscess, then some of the patient’s own white blood cells can be labelled with technetium-99m or with indium-111, which will accumulate in an abscess (Fig. 10.8).
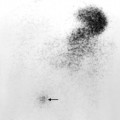
Fig. 10.8 Indium-111-labelled leucocyte scan of an intraperitoneal abscess. This abscess (arrow) followed small bowel surgery with subsequent anastomotic leak. Normal uptake is seen in the liver (L) and spleen (S).

RETROPERITONEUM
Computed tomography, MRI and ultrasound all provide information about retroperitoneal structures. Plain films are limited to showing: very large masses or calcification within a mass; the occasional case where gas is seen in an abscess; and the curvilinear calcification of an aortic aneurysm. Some of the differentials for retroperitoneal masses are given in Box 10.2.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree