Fig. 15.1
AMIGO suite
The presence of a PET/CT scanner in this suite will, for the first time, introduce MI into therapy and surgery. The integration of anatomical information from CT and MRI with the functional and metabolic information from PET will inform surgical decision-making during tumor resections and percutaneous thermal ablations. Using this technology, we envision that physicians can localize and target viable tumor tissue before a procedure and verify the completeness of surgical removal or destruction of tumors by depicting any residual cancer tissue before the end of the procedure. Using integrated patient transfer technology, the patient, as well as monitoring and anesthesia delivery equipment, can be efficiently moved from the PET/CT table to the operating/angiography room table. The marriage of a procedure room with an MI environment will accelerate the validation of new contrast agents, as well as enhance clinical decision-making.
MI-guided surgeries and interventions require validated MI imaging agents, and the presence of a PET/CT system in the AMIGO suite gives us a unique platform for in vivo human validation of these agents, at present primarily for brain and prostate cancer but eventually for other malignancies. Multiple pathological samples from open brain surgery or prostate biopsies will be acquired and compared to PET data from corresponding image locations. Very precise mapping of sampling sites to image locations will be possible. This capability will make possible the much-needed translational steps for the validation of new tumor localizing and characterizing MI agents.
Requirements for Quantitative PET Imaging
Recent advances in oncologic surgery, both traditional and noninvasive, and radiation therapy have increased the demands for quantitative capability of PET imaging, not only in terms of accuracy and precision but also in terms of spatial scale. MRI-guided open brain surgery [1], noninvasive MRI-guided ablation, by focused ultrasound [2] and other techniques, and targeted drug delivery through the blood–brain barrier [3], major initiatives of our center, depend critically on correct definition of tumor extent based on knowledge of tumor cell density and, in some cases, other pathological or functional measures, in the margins and, in some cases, within subvolumes of the tumor. The development of new PET tracers that are specific to various aspects of tumor biology or physiology has raised the possibility of a shift from anatomical imaging-based radiation therapy planning to MI-guided radiation therapy [4]. The concept of a “biological target volume” was introduced by Ling et al. [5], who pointed out the potential for exploiting newly available “metabolic, functional, physiological, genotypic, and phenotypic data,” along with the capability for individually tailored dose distributions by intensity-modulated radiotherapy, to achieve major advances in cancer management. Some centers are now incorporating “boosts” to tumor subvolumes on the basis of functional PET imaging [6–8]. Quantitative PET imaging is also crucial to treatment planning for targeted radionuclide therapy (TRT) [9] and dose verification of particle therapy [10].
Radiation oncology treatment planning can be viewed as a boundary (or series of boundaries)-definition task. The boundary to be defined might be, e.g., between tumor margin to be treated and normal tissue to be spared or between hypoxic areas to be treated to higher dose and areas of highly perfused tumor. The plan might require a series of boundaries delineating target isodose contours. Surgical planning and surgical decision-making also require the knowledge of boundaries. Although MRI with dynamic contrast enhancement defines the margins of the highly vascularized portion of the tumor, which contains abnormal blood vessels, it does not show the adjacent zone, in which tumor cells infiltrate normal tissue but do not induce angiogenesis. If the required boundaries are to be defined on the basis of PET imaging data, then knowledge of the accuracy and precision of the voxel activity estimates, as well as the covariance matrices, is crucial. With this knowledge, it is possible to determine confidence intervals on the boundaries using the approach of Hanson [11]. Better information on the limits of PET tumor imaging will result in more complete tumor treatments and enhanced effectiveness of image-guided therapies, leading to a significant improvement in patient outcomes.
Performance in definition of tumor extent is determined by the properties of both the tracer and the PET imaging system. The fidelity with which the tracer distribution (activity concentration) reflects, e.g., tumor density, constitutes a limit on accuracy, and the characteristics of the PET hardware and software determine the accuracy and precision of the voxel estimates of activity concentration. The relationship between tracer uptake and various pathologic measures will be determined using the AMIGO MI validation platform; multiple tissue samples through the tumor and its margins, obtained from glioma tumors during open brain surgery and from prostate tumors during image-guided biopsy, will be assessed for absolute activity concentration and analyzed pathologically. The second factor, i.e., the capability of the PET system to reproduce the tracer activity distribution, is best addressed by simulation and phantom experiments.
Validation of MI Agents: Tracer Distribution (Brain)
The Central Brain Tumor Registry of the USA estimates that each year more than 50,000 people in the USA are diagnosed with a primary brain tumor [12]. For most patients with brain tumors, neurosurgery is a key component of treatment [13, 14]. Neurosurgical resection achieves cytoreduction, decreases mass effect and attendant neurologic deficits, provides tissue for diagnosis and increasingly for prognosis, and maximizes the benefit of adjuvant treatment. Nevertheless, successful treatment of patients with primary brain tumors, even when gross total resection is achieved, is limited because surgery is not able to address the infiltrative portion of the tumor. Moreover, targeting of radiation therapy is uncertain since the full limits of tumor cannot be defined by conventional imaging, and chemotherapy delivery is limited by the blood–brain barrier. Thus, even with the therapeutic triad of maximal surgical excision, radiation therapy, and chemotherapy, the prognosis for most patients with primary brain tumors remains poor.
Maximal tumor resection, when it can be achieved without increasing neurologic morbidity [15], improves prognosis for patients with primary brain tumors. Claus et al. [16] showed that low-grade glioma patients who underwent partial resection had a 4.9 times higher risk of death than patients who underwent gross total resection; the extent of resection is also an important prognostic factor in high-grade gliomas. However, intraoperative determination of tumor extent is difficult because these tumors resemble brain tissue, and damage to critical brain tissue can lead to permanent disability or death. The addition of advanced structural and functional data to conventional imaging and neuronavigation can help surgeons with intraoperative decision-making regarding whether or not to resect tissue. For example, the addition of presurgical tractography to neuronavigation has been found to increase tumor resection and survival and decrease neurologic morbidity [17]. The majority of malignant gliomas recur within 2 cm of the enhancing edge of the original tumor [18], making the delineation of the tumor margins critical to clinical decision-making. The development of techniques capable of accurately depicting tumor margins in vivo is important for the determination of the optimal resection strategy, that which maximizes resected tumor while avoiding injury to adjacent brain. To date, intraoperative MRI has been the most widely adopted surgical adjunct to improve brain tumor resection; this approach, however, is time and resource intensive. Furthermore, while excision of the MRI-demonstrated abnormality is the accepted goal for surgical resection of brain tumors, there is a consensus that conventional MRI tends to underestimate the extent of the tumor. Thus, even radiographically “complete” resections leave variable numbers of tumor cells behind, leading to recurrence [19]. The intraoperative use of fluorescence markers to indicate residual tumor, though applicable primarily to high-grade gliomas, has proved effective in minimizing MRI-defined residual tumor and in improving clinical outcome (survival) [20]. Thus, there is support for the intraoperative use of an imaging biomarker to guide neurosurgery. However, the use of fluorescence is limited to open surgery; we would like to develop imaging approaches that can define the extent of tumor without direct visualization, i.e., without craniotomy.
Despite tremendous improvements in the ability to visualize brain tumors prior to surgery and to use imaging, both pre- and intraoperatively, to guide surgery for brain tumors, there remains great uncertainty in any given patient about the extent and degree of tumor infiltration. Tumor infiltration appears to follow the white matter fiber bundles, possibly along the vessel channels, without disrupting the blood–brain barrier. This is the reason that MRI may be inadequate as a stand-alone modality to predict the true extent of glioma and should be complemented by more sensitive modalities. The development of techniques capable of accurately depicting tumor margins in vivo is important for intraoperative decision-making to optimize resections in patients with gliomas. Defining imaging biomarkers that are able to demonstrate in three dimensions the presence and amount of tumor cells could also benefit radiation treatment planning and allow the development of novel therapeutic strategies such as blood–brain barrier opening for targeted drug delivery.
As a first step toward MI-guided surgery, we must validate both clinically available and investigative MI agents for the detection of primary malignant gliomas. In order to use surgical or biopsy samples for validating images or imaging agents, samples should be taken from multiple sites within and around the tumor boundaries. Single biopsies can be misleading because they are susceptible to sampling error due to tumor heterogeneity. Multiple biopsies via burr holes are not optimal in the brain due to the risk of hemorrhage; therefore, tissue samples will be taken during open brain surgery. We have shown that multiple biopsies taken during tumor resection have a range of tumor content (from low to high proportion of tumor cells relative to background brain tissue) [21] and can provide enough data for the validation of MI agents.
It is also crucial to accurately map the original in situ locations of the tissue samples to the PET and MRI images so that the corresponding imaging measures can be correlated with histopathologic data. Precise image-to-patient space registration of samples requires intraoperative imaging, because preoperative (or pre-biopsy) imaging would not reflect the significant shifts and deformations that occur during surgery (or displacements during multiple needle biopsies). Image-registered samples will be acquired in the multimodal surgical setting of AMIGO, where the intraoperative imaging systems, navigational tools, and registration methods developed at our center [1, 21, 22] can be used to match multiple pathology samples to corresponding imaging data with high accuracy (Fig. 15.2). From these data, we will determine the functional relationship between image-derived and tissue-derived parameters.
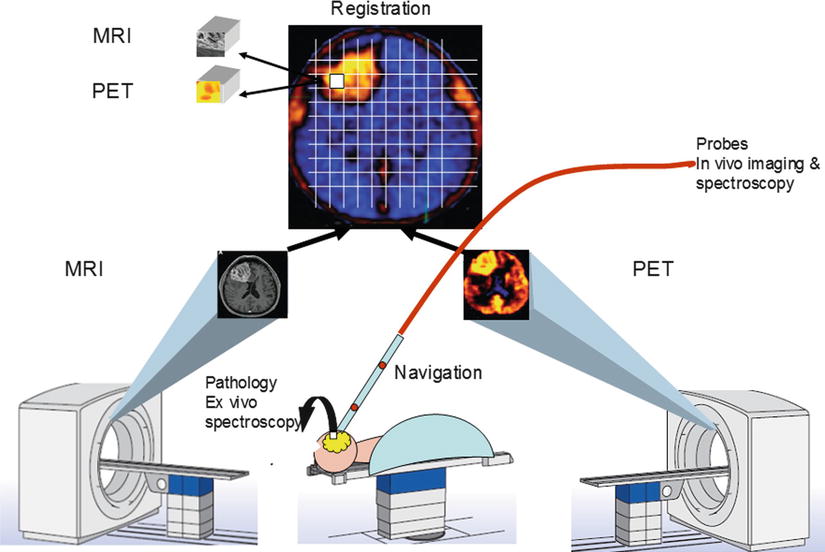

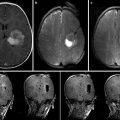
Fig. 15.2
Diagram of AMIGO suite with MR and PET/CT systems illustrating brain imaging and tissue validation for glioblastoma
We have developed Slicer modules that can integrate multimodality preoperative images into commercial navigation systems [23]. We have also successfully deployed this software and hardware into the neurosurgical operating room [24]. Building on these advances, we will develop an intraoperative Slicer module which will be able to receive, store, coregister, and annotate multimodality images from multiple time points during the operation. We have used a similar approach for the localization of biopsy samples for study with mass spectrometry, though that work was limited to annotating preoperative images, as the AMIGO suite was still under construction [21]. A mass spectrometer has been installed in the AMIGO suite, and it will be used for the intraoperative analysis of tissue samples from brain tumor and breast cancer surgeries.
Validation of MI Agents: Tracer Distribution (Prostate)
Our goal is to determine the capability of imaging systems to provide an in vivo map of focal prostate tumors, thereby serving as a surrogate for saturation biopsy. Although our primary interest is planning for focal therapy, the information we gain may be valuable in other clinical prostate cancer situations, e.g., for active surveillance or planning biopsies in men with PSA abnormalities. The prostate biopsy will provide the direct tissue validation for PET imaging. It is well documented that current methods of detecting cancer are far from perfect. The value of PSA screening has recently been revealed to be limited; it appears to be of little value in saving lives. The recently published PCLO trial showed that there was, essentially, no benefit to PSA screening [25]. The data also implied that to save one man’s life from prostate cancer, 43 other men must undergo unneeded biopsies and treatments, with associated side effects. Thus, the current practice of overdiagnosing prostate cancer due to the widespread application of TRUS biopsy has led to overtreatment of prostate cancer becoming a major public health problem.
As a first step toward MI-guided prostate cancer treatment planning, we must validate both clinically available and investigative MI agents for the detection and characterization of focal prostate cancer. Diagnostic biopsies typically involve multiple samples (12–15 cores) that are intended to systematically sample the prostate. In selected situations, e.g., multiple prior negative biopsies or therapy planning, a saturation or “mapping” biopsy can be performed. These multiple biopsy samples, usually 40–60 cores taken at 5-mm increments through the gland, can provide adequate data for validation of MI agents used for detection and 3D mapping of prostate cancer. These samples are expected to cover the full range of tumor biological metrics and will include normal tissue. Our planned correlation of radioactivity concentration with histological metrics on a very fine spatial scale (sample volume ~ 1.5 mm3 for 18F-labeled tracers) will yield the most direct validation of PET radiotracers possible.
We believe that the AMIGO MI validation platform will provide a unique and crucial resource for the validation of many MI biomarkers. The ability to visualize specific tumor characteristics, such as proliferation status, will make possible distinct characterization of different areas within the same tumor. The MI agent validation platform will provide the currently missing link that is necessary for scientifically rigorous translation of newly developed MI agents to clinical use.
At the Brigham and Women’s Hospital, we began MRI-guided prostate biopsies in 1997 in our intraoperative double-donut open 0.5T (GEMS Signa SP) MRI environment (see Chap. 56 on Image-Guided Prostate Interventions). This work established the technical and clinical feasibility for MRI-guided biopsy. We also performed MRI-guided prostate brachytherapy in the same device. Over 700 men with localized prostate cancer had brachytherapy implants using MR to define the clinical target volume (CTV). The CTV depended upon the MR to define the peripheral zone of the prostate, which was to receive the maximum 125I dose. In 2010, we relocated our prostate biopsy program to the new wide-bore 3T MR system in AMIGO, which provides greatly improved signal-to-noise ratio and the possibility of transperineal sampling in the bore of the magnet.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree