CHAPTER 34 Pineal Region Masses
The pineal gland is a unique neuroendocrine structure situated roughly in the center of the skull. It has been the subject of interest and speculation by anatomists and natural philosophers since ancient times, with references as far back as 800 BC.1 Its modern name is based on its pine cone–like shape, and, for the same reason, the pineal was previously referred to as the conarium.1 Historical theories detailing its function and purpose abound. As early as 300 BC, Herophilus and others believed that it was the seat of the mind and functioned as a sphincter to regulate the flow of thoughts.1 Galen (circa AD 150) was convinced that it was a gland and proposed that the cerebellar vermis was actually the thought sphincter.1 The noted philosopher and natural scientist René Descartes (c. 1750) was keenly interested in the pineal gland. Despite objections from his contemporaries such as the Dutch anatomist Caspar Bartholin, Descartes revived the concept that the pineal gland was the seat of the soul in his work The Passions of the Soul (1749).1 François Magendie and other physicians of the same period resurrected the ancient notion that the pineal gland was a sphincter to control the flow of cerebrospinal fluid (CSF) rather than the flow of thought.1 In 1763, Thomas Gibson in his Epitome of Anatomy described the pineal body as an endocrine gland, which leads us into present-day concepts.1
OVERVIEW
Embryogenesis and Maturation
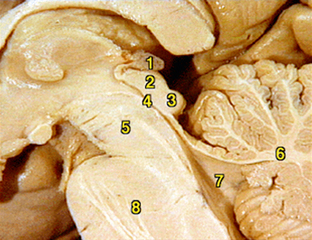
The pineal gland, or epiphysis cerebri, evaginates from the posterior diencephalic roof during early embryonic life.2 The mature gland hangs by the pineal stalk from the posterior roof of the third ventricle (Fig. 34-1). Within the pineal stalk is the pineal recess,2 a passageway lined by ependymal cells and directly connected to the third ventricle. The gland is fully formed by around 1 year of age and gradually increases in size to age 2 years, after which it remains stable until age 20 years.3 Throughout adult life there is a gradual decrease in melatonin synthesis and release, and gradual involution may occur, although this is variable.4 Pineal parenchyma typically contains a variable quantity of calcific particles, called corpora arenacea (“brain sand”). Chemical analysis shows that these structures are composed of calcium phosphate, calcium carbonate, magnesium phosphate, and ammonium phosphate. As the gland ages and involutes, cysts occur within the pineal with increasing frequency. Most cysts measure less than 1 cm, with some larger cysts measuring up to 3 cm. Up to 40% of glands show various-sized cysts at autopsy, and a recent high-resolution MRI study showed pineal cysts in 23% of normal adults.5 Some studies have shown slightly increased incidence of pineal cysts in females. Pineal cysts may remain stable, or they may increase or decrease in size over time.6 Most pineal cysts are considered incidental and nonpathologic unless they exhibit untoward mass effect or other atypical features, such as hemorrhage. A recent study found a higher than predicted association between incidental-appearing pineal cysts and history of headache, although the existence of a causative etiology is lacking.7
Histology
Histologically, the pineal gland contains two main cell types. Primary pineal parenchymal cells are of a similar lineage as the photosensory cells of the retina, which display further evolution into neurosecretory cells. Combined immunohistochemical and ultrastructural data corroborate the dual neurosensory and neurosecretory nature of pineal parenchymal cells. Parenchymal cells stain positively for both synaptophysin and neurofilament protein. These specialized neurosecretory cells are contained within a multilobular and septated structure composed of leptomeningeal and glial elements. Glial cells within the pineal gland are primarily astrocytic, but ependymal elements and possibly choroidal cells may be present in fewer numbers. The cellular development of the human pineal gland recapitulates its development as a neurosecretory organ derived from photosensitive cells. In late intrauterine life, the gland is composed primarily of pigmented cells arranged in a rosette pattern similar to the developing retina. In subsequent postnatal development, pineal cells lose their pigmented components and develop positive reactivity for neuron-specific enolase.8
The external capsule of the pineal gland is leptomeningeal, of primarily arachnoidal tissue, and continuous and commingled with internal glial elements. There is extensive sympathetic innervation via the superior cervical ganglion with some parasympathetic innervation. True ganglion cells are present but are rare within the pineal gland. These cells rarely give rise to a true primary pineal ganglioglioma. Finally, the pineal gland is permeated by a dense capillary bed of vessels, without forming a blood-brain barrier.1,9
Pathophysiology
The most important function of the pineal gland is the elaboration and secretion of melatonin. Melatonin has at least two distinct functions. First, it regulates our diurnal cycle, whereby bodily functions are organized around a day-night schedule. The production of melatonin is stimulated by darkness and inhibited by light. How does this occur? In submammalian species, the pineal gland is a light-sensitive organ and has been referred to as the “third eye,” which is thought to be related to the parietal eye of some ancient and primitive fish species. In some reptiles and amphibians, the gland is close to the skin surface in the back of the neck and receives direct stimulation from light that penetrates superficial tissues.10 In the human, the pineal gland receives no direct photic input but retinohypothalamic tracts transmit light/dark information to the suprachiasmatic nucleus and then to the tuber cinereum of the hypothalamus. This information is subsequently conveyed in the medial forebrain bundle, leading into the inferomedial column of the spinal cord and thence to the superior cervical ganglion. Sympathetic fibers from the superior cervical ganglion follow the carotid vessels upward to the parasellar region and then track posteriorly to provide direct sympathetic input to the pineal gland (Fig. 34-2). Because of this circuitous pathway, the pineal gland is referred to as a neuroendocrine transducer, which transforms retinal light/dark signals into neuroendocrine secretions, in turn regulating other neural and somatic functions.9 Abnormal melatonin production and release is implicated in a variety of conditions, including seasonal affective disorder, jet lag, narcolepsy, and other sleep disorders.11
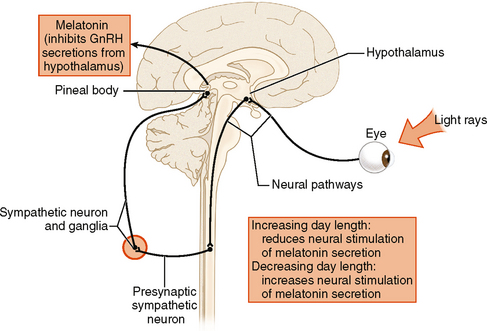
FIGURE 34-2 Schematic drawing of the pathway of light, which is converted to photic impulses that travel from the retina to the superior chiasmatic nucleus (SCN) down the brain stem into the cervical cord, out along sympathetic fibers to the superior cervical ganglion, and then back superiorly to the pineal gland. It is by this pathway that day-night cycles are “read” by the pineal gland, which then modulates the release of melatonin, creating our physiologic diurnal cycle.
Epidemiology
Tumors in the pineal gland and adjacent region have historically been grouped together and at one time were collectively referred to as pinealomas. With better understanding, this term has been abandoned in favor of the more general term pineal region masses. These entities fall into four major categories: germ cell tumors, pineal parenchymal tumors, gliomas, and other neoplastic and non-neoplastic masses or cysts. Accurate epidemiologic information about pineal tumors is problematic owing to variations in classification and regional differences in biopsy protocols. Because germinomas, the most common tumor in this region, are exquisitely radiosensitive, many pineal region tumors have historically been treated with primary radiation therapy without histologic confirmation. Although this practice has produced good clinical results, it has made it more difficult to ascertain the true incidence of different tumor types. Large series historically suggest an overall incidence of pineal region tumors of 0.4 to 0.9 per 100,000/year,12 which represents less than 1% of all new intracranial tumors per year in adults and between 3% and 8% of new pediatric brain tumors.13 Of these, germ cell tumors are most common, accounting for 50% to 60% with an estimated incidence of approximately 0.4 per 100,000/year in the United States and Europe.14 Next most common are tumors of pineocyte origin, which account for approximately 14% to 27% of pineal region masses,15,16 with an estimated incidence of 0.1 to 0.2 per 100,000/year, representing 0.3% to 0.6% of new brain tumors in adults and 3% to 4% in children.17
Pineal region tumors have been found to exhibit significant geographic variation for reasons that are not known. There is a significantly increased incidence of germ cell tumors in Japan and to a lesser extent elsewhere in Far East Asia, with estimated three to four times as many germinomas as in the United States.17 Although data are incomplete, true pineal parenchymal tumors do not appear to have major geographic variations in incidence.
Overall, pineal region tumors are more common in younger age groups. Germ cell tumors taken as a group have an average age at onset of 11 years. Pineal parenchymal tumors occur somewhat later and display a wider age range, with mean ages in different series from 22 to 36 and an age range between childhood and the eighth decade of life.15
Germ cell tumors of the pineal region are approximately three times more common in males,18 although this figure is even higher in some series; however, in the suprasellar region this gender differential is not seen. Pineal parenchymal tumors do not display a convincing gender predilection overall, although a slight male bias has been reported in Japan.17
Of germ cell tumors, germinomas are most common, representing more than 50% in most series. Teratomas are next most common, representing 15% to 20% of intracranial germ cell tumors, followed by small numbers of endodermal sinus or yolk sac tumors, choriocarcinoma, and embryonal cell carcinoma, each of which contributes 3.5%, with the remaining 10% to 15% comprising germ cell tumors of mixed histology (Fig. 34-3).15
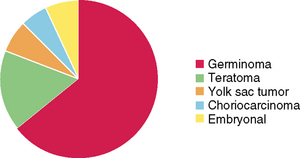
FIGURE 34-3 Relative proportions of different types of intracranial germ cell tumors from the Armed Forces Institute of Pathology.
The histogenesis of intracranial germ cell tumors is unknown. It is hypothesized that primitive cells, which have already begun germ cell development along the urogenital ridge, are accidentally enfolded into the neural tube and subsequently become neoplastic.18 Earlier and less differentiated embryonic germ cells may give rise to biologically more aggressive tumors. Why does this development of germ cell neoplasms occur specifically and most commonly in the pineal region and secondarily in the hypothalamic pituitary region? Although undoubtedly multifactorial, with some genetic factors playing a role, the production and storage of pineal melatonin and hypothalamic gonadotrophins in these regions is believed likely to play some role in their pathogenesis.15
Pineal parenchymal tumors comprise slightly less than a third of total pineal region masses and are divided between pineocytomas, 40%; pineoblastomas, 30%; and the rest with mixed pineoblastoma/pineocytoma histology. Pineal parenchymal tumors have an average age at onset somewhat later than germ cell tumors, with 22 years for pineoblastoma, 32 years for pineocytomas, and 36 years for mixed lesions.17
Clinical Presentation
Clinically, pineal region tumors or masses may present as a variety of signs and symptoms due to mass effect on adjacent structures. One common manifestation of pineal region masses is Parinaud’s syndrome, first described by Henri Parinaud, an ophthalmologist working with Jean-Martin Charcot in Paris in the late 1800s. This syndrome is also referred to as pretectal syndrome, dorsal midbrain syndrome, and other less common eponyms. It is produced by downward mass effect of the pineal region on the tectum of the midbrain, which produces a variety of neuro-ophthalmologic symptoms due to oculomotor tracts passing through this region. Common symptoms include paralysis or paresis of upward gaze from impingement on the rostral medial longitudinal fasciculus and/or posterior commissure, eyelid retraction (Collier’s syndrome) possibly resulting from excess superior rectus innervation, and levator palpebrae innervation, convergence retraction nystagmus, and impaired pupillary reaction to light sometimes with sparing of near light reactivity. Other less common findings include intermittent esotropia due to pseudoabducens palsy and convergence and accommodation insufficiency.19
In addition to neuro-ophthalmologic manifestations, generalized signs of increased intracranial pressure and hydrocephalus may be present due to obstruction of the aqueduct of Sylvius. These include headache, syncope, and other generalized symptoms. Mass effect on or invasion of the hypothalamic pituitary axis or impingement on the pineal gland itself may disrupt the normal regulation of melatonin production and secretion, producing precocious puberty, diabetes insipidus, and other endocrinopathies. Precocious puberty, in particular, is more commonly associated with germinomas and other germ cell tumors; this may be due to decreased circulating melatonin as well as increased gonadotropins such as human chorionic gonadotropin (hCG) secreted by the tumor.
GERMINOMA
Germinomas are intracranial tumors of germ cell origin. They are exquisitely radiosensitive neoplasms, with up to 80% to 90% curable by radiation therapy alone.20 Therefore, noninvasive diagnosis, through a combination of imaging and serum and CSF analysis accompanied by clinical evaluation, is of vital importance. If noninvasive evaluation is not definitive, and tissue diagnosis is required, ventriculoscopy may provide a less invasive biopsy approach. This technique has been shown to provide adequate tissue sampling and allows for placement of a definitive ventriculostomy for treating hydrocephalus and even tumor debulking.
Epidemiology
Germinoma is the most common type of intracranial germ cell tumor, representing 50% to 60% in most series. These are usually midline tumors, with approximately 80% of germinomas arising in the pineal region and most of the remaining examples in the suprasellar or parasellar regions. Only rarely do they present elsewhere in the brain.21 The average age at presentation is 11 years, and in the pineal region there is a marked male predominance. The male-to-female ratio of pineal region germinomas is approximately 2.5 : 1 overall, although much higher percentages of up to 4 to 5 : 1 or more have been reported in the Japanese population. In the United States, the incidence is estimated at 0.1 per 100,000/year,22 whereas this may be twice as high in Japan. Significant differences in the incidence of pure germinomas have been reported in several large studies, with the greatest incidence reported in Japan, Korea, and the Republic of China.23
As noted, 10% to 20% of germinomas arise in the sellar region, and in this location there is no clear male predominance. Various studies have reported either an equal male-to-female ratio or a slight female predominance. In addition, a small number of germinomas are found scattered elsewhere throughout the neuraxis (Fig. 34-4).
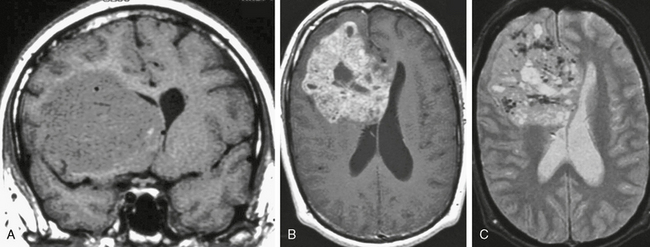
FIGURE 34-4 Hemispheric germinoma in a 37-year-old man. The majority of intracranial germinomas occur in the pineal region, with a secondary locus in the pituitary hypothalamic region. A very small percentage, less than 5% of the total, occurs in other locations, including the skull base and within the cerebral hemispheres, as in this case. On coronal precontrast (A), axial postcontrast (B), and axial T2W (C) MR images there is a large, complex, solid and fluid mass with mass effect on the frontal horns and dense enhancement of the solid components. This imaging appearance is relatively nonspecific but on pathologic examination displayed typical histology for germinoma.
Clinical Presentation
Diabetes insipidus may occur due to extension and impingement on the hypothalamic region and pituitary stalk, resulting in suppression of arginine vasopressin, also known as antidiuretic hormone. Increased serum and CSF levels of placental alkaline phosphatase (PLAP), β-hCG, and α-fetoprotein (AFP) are frequently present in blood and in CSF and serve as diagnostic markers and prognostic indicators.24
Pathology
Pathologically, germinomas are typically firm, well-circumscribed solid masses that are whitish or tan (Fig. 34-5). Fluid (“cystic”) regions are seen in up to one third of cases, particularly in larger tumors. Germinomas, although usually round when small, preferentially grow in several directions, including inferiorly and posteriorly into the posterior fossa, superiorly and anteriorly into the third ventricle, and anteriorly and inferiorly into the hypothalamic, chiasmatic, and suprasellar regions.
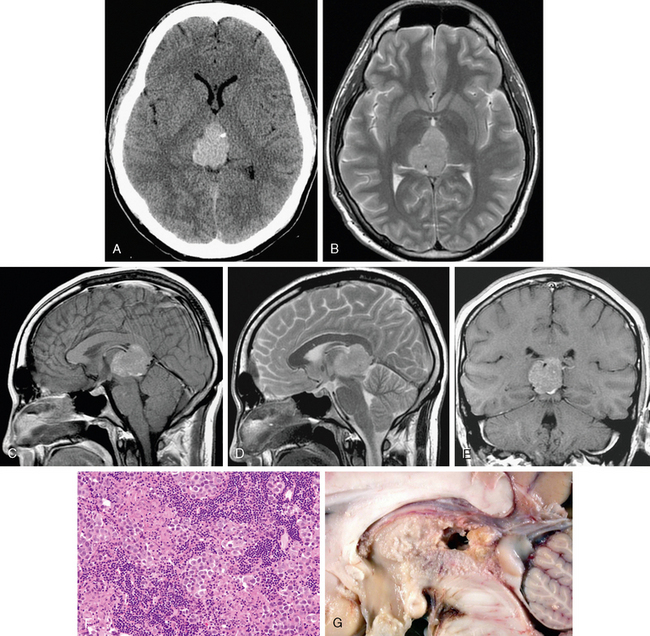
FIGURE 34-5 Pineal germinoma. A rounded but slightly lobular mass is shown that is hyperdense on noncontrast CT, with diffuse moderate homogeneous signal intensity and enhancement on MRI. A, CT image without contrast. B, Axial T2W MR image. C, Sagittal T1W MR image after gadolinium administration. D, Sagittal T2W MR image. E, Coronal T1W MR image after gadolinium administration. F, Histologic preparation shows typical biphasic histology of round mononuclear tumor cells engulfed in an inflammatory response consisting mainly of small lymphocytes, primarily both helper and killer T cells. G, Gross anatomic specimen shows relative location to brain stem and corpus callosum.
The lymphocytic component is primarily T cell, including both helper and suppressor subtypes (see Fig. 34-5).
Immunohistochemical analysis shows OCT4 reactivity as the most helpful marker, followed by CD117 and PLAP.25
Imaging
CT
On CT, the typical appearance of a germinoma is that of a hyperattenuating mass in the pineal region that typically surrounds or “engulfs” the gland and any pineal calcification that is present (Fig. 34-6). Cysts are present in a third to a half of cases and are more clearly observed in larger tumors (Fig. 34-7). Dense uniform enhancement of the solid component is typical.26
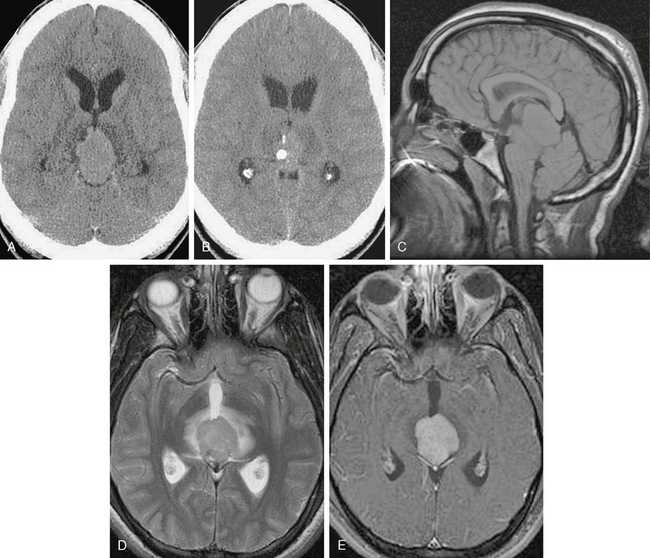
FIGURE 34-6 Germinoma. On initial CT (A, B) there is a soft tissue mass surrounding and engulfing a coarse chunk of calcification. This pattern is considered typical for germinoma, which arises outside the densely calcified pineal parenchyma. C, Sagittal precontrast T1W MR image shows mass effect with inferior cerebellar tonsillar excursion through the foramen magnum. D, Axial T2W MR image shows the mass with surrounding edema. E, On postcontrast T1W MRI there is dense uniform enhancement. Note that the calcification clearly seen on CT is not detected by MRI.
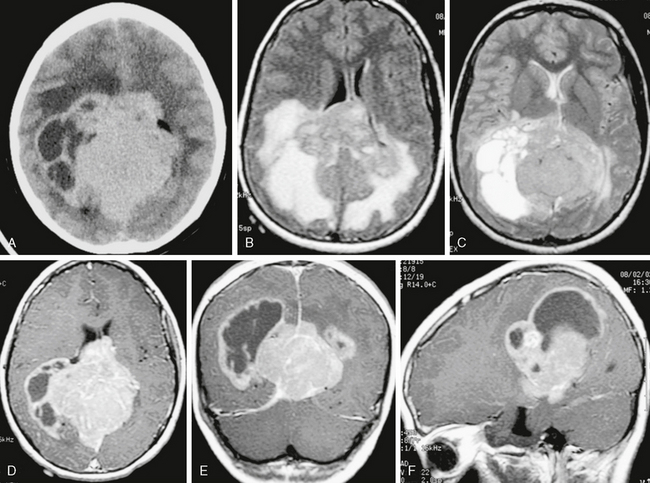
FIGURE 34-7 Large solid and cystic mass roughly centered on the pineal region in a 16-year-old boy. Selected images show irregular solid components that are hyperdense on noncontrast CT and relatively homogeneous in signal intensity and enhancement on MRI, with large cystic or fluid regions surrounded by enhancing capsule. A, Noncontrast CT image. B, Axial FLAIR MR image. C, Axial T2W MR image. D to F, Axial, coronal, and sagittal T1W MR images after administration of gadolinium.
MRI
On MRI, germinomas are typically homogeneous or partially “cystic” with signal intensity isointense to white matter on T1-weighted (T1W) images and slightly hyperintense on T2-weighted (T2W) images. After gadolinium administration, dense homogeneous enhancement is seen. Hemorrhage is uncommon in germinoma, as compared with other germ cell tumors.27 Because any CSF dissemination must be detected, postcontrast imaging of the entire neuraxis is mandated once a primary tumor is discovered. Direct extension, either into the posterior fossa, the third ventricle, or the hypothalamic pituitary region, may also be seen, particularly with larger tumors.
TERATOMA
Epidemiology
Teratoma is the second most common intracranial germ cell tumor, representing 15% to 20% of all intracranial germ cell tumors in most series. Teratomas are subcategorized as mature, immature, and malignant. Mature teratomas are most common, accounting for two thirds of all types, followed by immature teratomas. Malignant degeneration in a mature teratoma occurs much less commonly. Average age at diagnosis of a teratoma is 5 years, with a significant number presenting in the first year of life, or even on prenatal imaging studies such as ultrasonography or fetal MRI (Figs. 34-8 to 34-10). Teratomas are the most common intracranial tumor detected on prenatal imaging, either ultrasonography or fetal MRI. As with other germ cell tumors, there is a male predilection and the incidence is slightly higher in Japan, Korea, and the Republic of China.
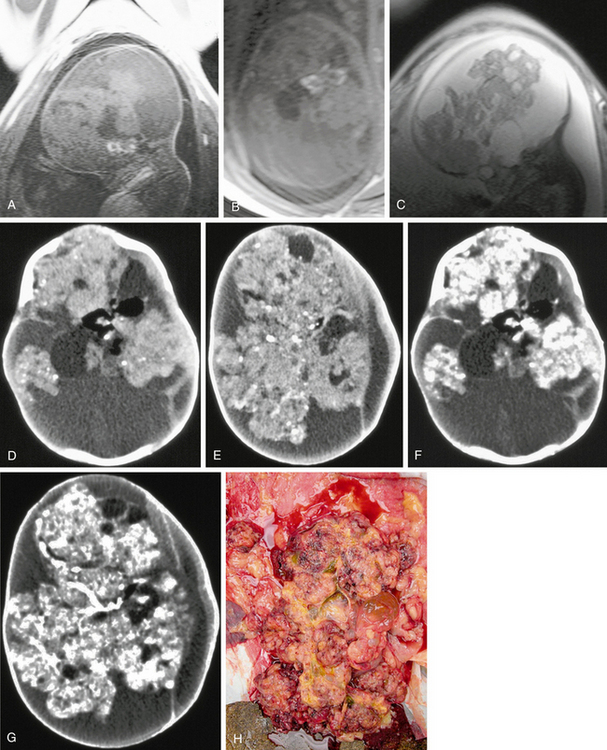
FIGURE 34-8 Large teratoma imaged and diagnosed prenatally on fetal MRI. A and B, Sagittal and axial T1W fetal MR images show bright signal indicating fat. C, Sagittal T2 fetal MR image. D to G, Postnatal CT scans before and after administration of a contrast agent show fat density and areas of solid enhancement. H, Gross pathologic specimen.
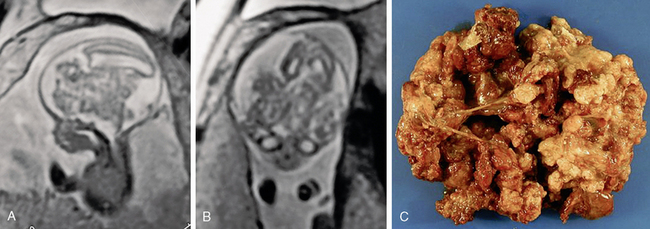
FIGURE 34-9 Teratoma. Intrauterine fetal sagittal (A) and coronal (B) T2W MR image shows hydrocephalus and a large irregular central mass. Subsequent pathologic evaluation provides a diagnosis of teratoma. C, Gross pathologic specimen.