Postoperative Imaging of Traumatic Brain Injury
6.1 Introduction
As described in earlier chapters of this book, traumatic brain injury (TBI) is a major problem in the United States, with 1.5 million head injuries occurring every year. Furthermore, it results in 52,000 deaths per year,1 with a preponderance of cases occurring in children and younger adults. As a leading cause of death and disability in this younger population, TBI results in a major expense to society. Reducing the degree of disability can have a significant financial impact, reducing both health care costs and resulting in an earlier return to the work. In the past, there was a lack of enthusiasm by many surgeons for operative treatment of TBI, as many believed prognosis was dictated by the inciting incident. An overall lack of high-quality, controlled research in the subject likely contributed to this pessimistic view. However, more recent data have shown that the mortality rate is significantly reduced at centers with aggressive treatment for TBI.2 An extensive literature review led to the third edition of the “Guidelines for the Surgical Management of Traumatic Brain Injury” in 2006, a joint venture between the Brain Trauma Foundation and the Congress of Neurological Surgeons, which helped outline indications for surgical intervention of TBI.3
Given the impact of aggressive treatment and early intervention on the morbidity and mortality rates of TBI, it is paramount that radiologists are familiar with indications for surgery, common surgical interventions, expected postsurgical appearance, and potential complications. The radiology literature focusing on postoperative imaging for TBI patients is scarce. In this chapter, we summarize the imaging findings of various surgical interventions and the expected and unexpected imaging findings of which radiologists, intensive care specialists, or surgeons must be aware.
6.2 Intracranial Pressure Monitors and External Ventricular Drains
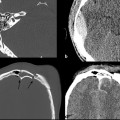
Although some patients with moderate or severe TBI are rushed immediately to the operating room, many others will first undergo a trial of nonoperative management, which may include bedside placement of intracranial pressure (ICP) monitors or ventriculostomy catheters. ICP monitor placement is usually indicated for TBI patients with a Glasgow Coma Score (GCS) of less than 8 (i.e., patients who are comatose after their initial injury). They are also placed in patients with a more mildly decreased score (9–12) who may be undergoing procedures that have the potential to affect the ICP.4 Placement can be done at the bedside in the intensive care unit, and they are usually inserted via a transfrontal approach after burr hole placement (▶ Fig. 6.1). Intraparenchymal hemorrhage at the insertion site is a fairly common occurrence (▶ Fig. 6.2), seen in almost 10% of patients at the authors’ institution.4
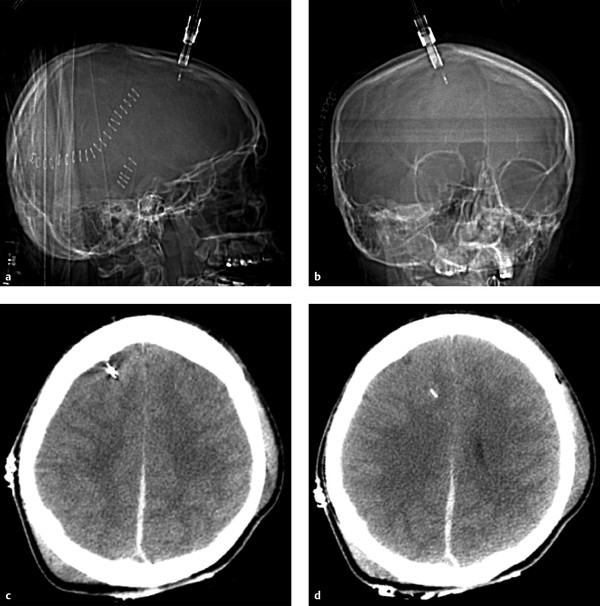
Fig. 6.1 Normal intracranial pressure (ICP) monitor placement. (a,b) Lateral and frontal computed tomographic scout images show the ICP monitor entering in the right frontal region. (c,d) Sequential noncontrast CT images in the same patient show a right frontal approach ICP monitor terminating in the frontal lobe. Parafalcine subdural hematoma is also noted.
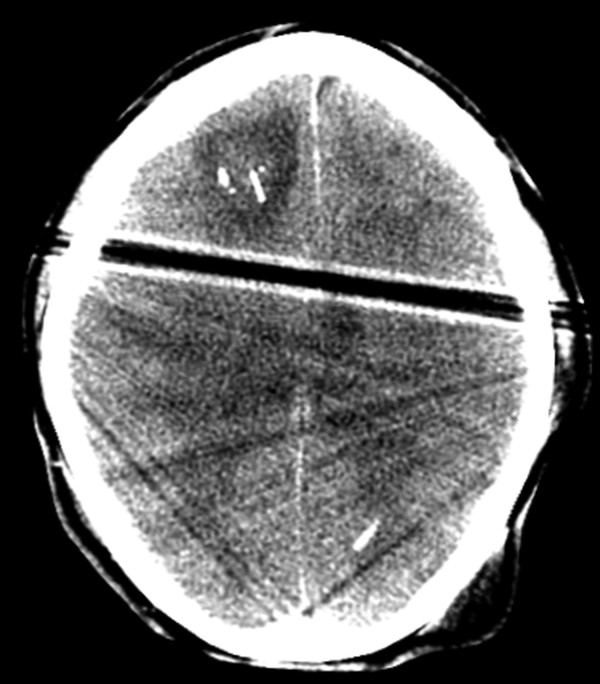
Fig. 6.2 Axial computed tomography image shows extensive edema and a small amount of hemorrhage surrounding a right frontal approach intracranial pressure monitor.
An external ventricular drain (EVD), or ventriculostomy catheter, can also measure ICP but has the advantage of also being able to lower ICP if necessary by draining off cerebrospinal fluid (CSF). An EVD can be placed at the bedside or in the operating room as part of a more extensive procedure. An EVD is most commonly placed via a transfrontal approach, entering the ventricular system at the frontal horn of the lateral ventricle and terminating midline near the foramen of Monro (▶ Fig. 6.3). However, placement can be complicated by concomitant intracranial injuries, as midline shift related to intracranial mass effect may be present, or the ventricles may be slit-like as a result of cerebral edema or swelling.2 Follow-up computed tomographic examinations should be evaluated for evidence of hemorrhage along the catheter tract (▶ Fig. 6.4) or for suboptimal catheter tract placement (▶ Fig. 6.5), both possible complications to the procedure.
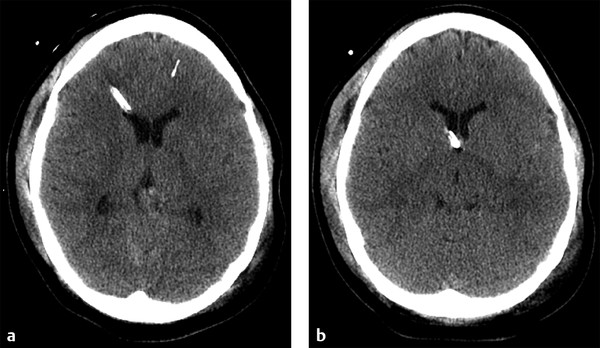
Fig. 6.3 Normal external ventricular drain placement. (a,b) Sequential noncontrast computed tomography shows a right frontal approach intracranial pressure monitor traversing the frontal horn of the right lateral ventricle, terminating near the foramen of Monro.
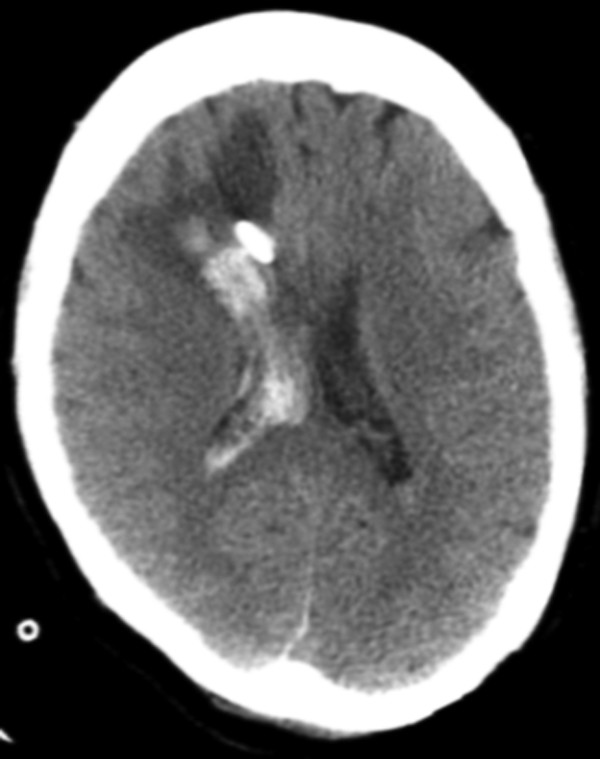
Fig. 6.4 Axial computed tomographic image shows a large amount of edema and hemorrhage surrounding a right frontal approach external ventricular drain in an area that was previously normal by imaging. Hemorrhage extends into the right lateral ventricle. The patient was status postsuboccipital craniectomy for hematoma evacuation.
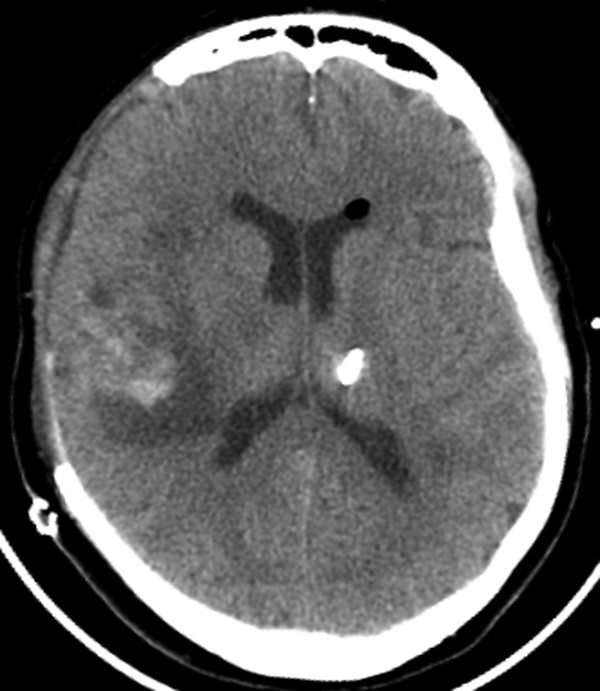
Fig. 6.5 In a patient status postdecompressive right craniectomy, there is suboptimal placement of a left frontal approach external ventricular drain, with the tip terminating in the left thalamus.
6.3 Subdural Hematomas
Acute subdural hematoma (aSDH) is one of the most common reasons for a TBI patient to undergo surgical intervention. Acute subdural bleeds warrant surgical intervention when they are larger than 10 mm thick or cause more than 5 mm of midline shift.2 For purely aSDHs, surgical intervention typically involves craniotomy for evacuation. As aSDH often extends over much of one hemisphere, large frontotemporoparietal craniotomies are typically performed for evacuation. Imaging after successful surgery typically shows a significant reduction in the size of the collection and adjacent mass effect, with some expected postcraniotomy changes, including gas, fluid, and a small amount of blood products at the craniotomy site (▶ Fig. 6.6).5,6
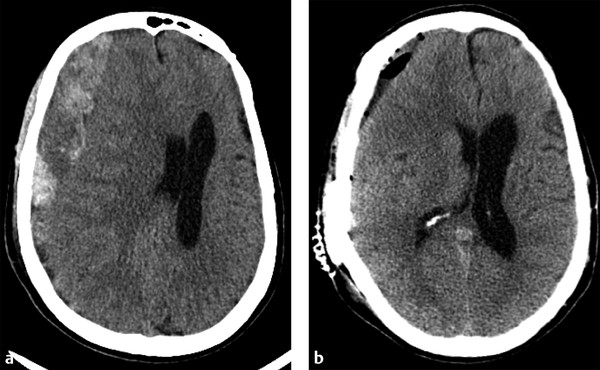
Fig. 6.6 Acute subdural hematoma (aSDH) evacuation by craniotomy. (a) Preoperative computed tomography shows a very large, predominately right frontal aSDH causing marked adjacent mass effect on the right cerebral hemisphere and right lateral ventricle, resulting in subfalcine herniation. (b) Immediately after aSDH evacuation by craniotomy, mass effect and leftward midline shift are markedly improved. Expected postsurgical changes include extra-axial gas, fluid, and minimal residual blood products at the craniotomy site.
Treatment of chronic SDHs, as well as acute on chronic SDHs, can be somewhat more complicated. Conservative management may be used for patients with asymptomatic collections with little adjacent mass effect.2 Given that chronic SDHs often occur in an older population in whom diffuse cerebral volume loss is more prevalent, even moderate-sized collections can have little mass effect on the adjacent brain parenchyma. When treatment is warranted, burr hole drainage is often the first step. Typically, two burr holes are created over the thickest part of the collection, and a drainage catheter is often left temporarily in place. Early imaging often shows only a mild reduction in collection size, with gas replacing some of the previously seen fluid. Complete resolution of the collection and adjacent mass effect often takes weeks to months (▶ Fig. 6.7). For collections that continue to recur after drainage, or for particularly large chronic SDHs, craniotomy may be used, similar to that done for aSDHs.2 In these cases, prior burr holes may be incorporated into the craniotomy.
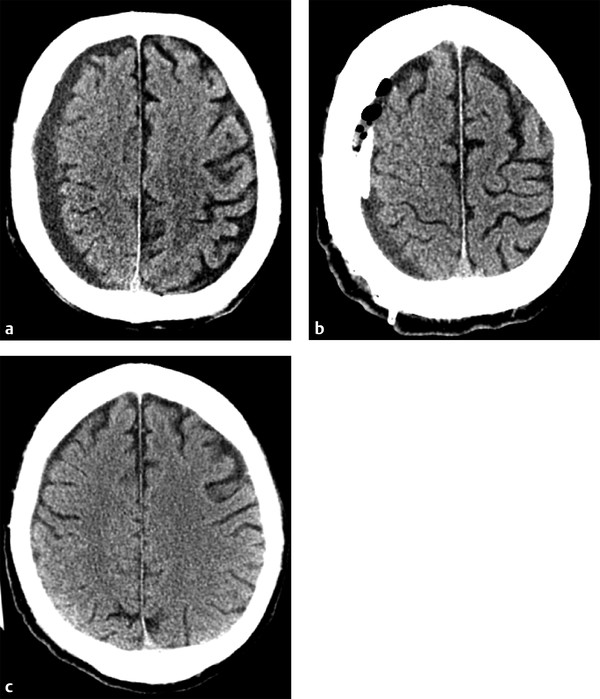
Fig. 6.7 Chronic subdural hematoma (SDH) with delayed improvement post burr hole drainage. (a) Presenting computed tomography shows a small-to-moderate sized chronic right holohemispheric SDH, with only mild adjacent mass effect given the underlying volume loss. (b) Immediately after Burr hole placement, the collection is only mildly decreased in size, with some of the hypodense fluid replaced by gas. A drain was left in the collection. (c) Follow-up head computed tomography performed 6 weeks later shows complete resolution of the SDH.
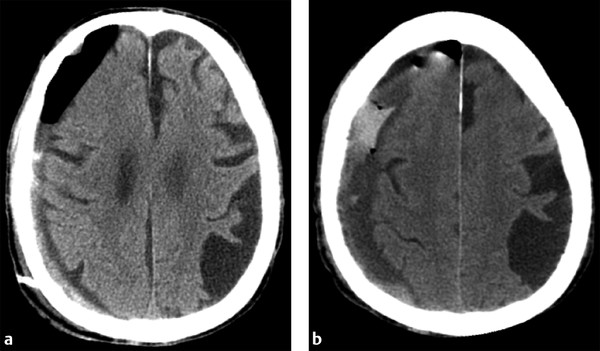
When evaluating postoperative imaging studies after either acute or chronic SDH evacuation, attention should be given to rebleeding, which can appear as either an increase in size of the collection or increase in collection density (▶ Fig. 6.8; ▶ Fig. 6.9). Reducing the collection and adjacent mass effect can result in blossoming of other intracranial injuries, such as parenchymal contusions and contralateral subdural collections, and follow-up imaging may demonstrate interval enlargement (▶ Fig. 6.10; ▶ Fig. 6.11).
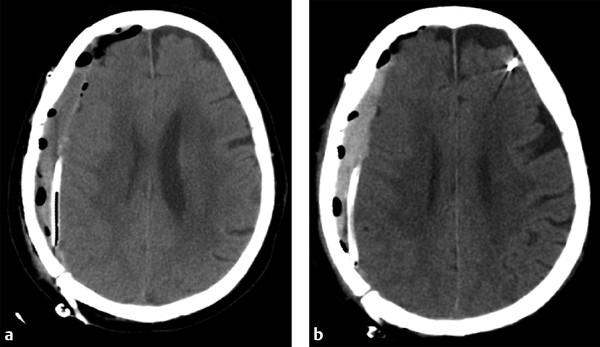
Fig. 6.8 Rebleeding following craniotomy for SDH evacuation. (a) Postcraniotomy computed tomography (CT) shows a small residual right holohemispheric SDH with expected postoperative gas. A drain was left in place. (b) Follow-up CT shows little change in size to the residual collection, although the collection is now hyperdense, suggestive of rebleeding.