Fig. 6.1
Glial fibers intermixed with a rich vascular network
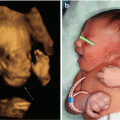
Its diagnosis is rarely described prenatally; in cases reported, the finding was usually during the second or third trimester when ultrasound demonstrated a voluminous mass extending from the internal canthus to the nasal bridge [9–12] (Figs. 6.2, 6.3, and 6.4).
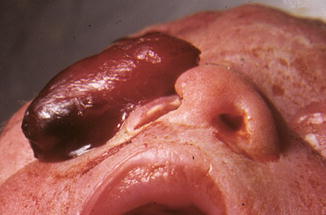

Fig. 6.2
Three-dimensional ultarsond in third trimester with surface rendering mode showing a circular mass at the level of the left nasal ala

Fig. 6.3
Intraoperative finding in the same case

Fig. 6.4
Large nasal glioma in a postmortem specimen demonstrating a large nasal glioma involving the right nasal ala
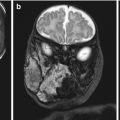
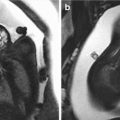
Postnatally, CT scan is often the initial imaging study obtained because it provides good visualization of the bony landmarks of the skull base. MRI has better soft tissue resolution and may be the best initial study in patients seen early in life because the anterior skull base consists of unossified cartilage and may falsely appear as if there is a bony dehiscence on computed tomography [8]. T1-weighted imaging demonstrates the mass to be isointense to gray matter with moderate enhancement with contrast [10]. On T2-weighted imaging, these lesions appear hypointense [13] (Fig. 6.5).
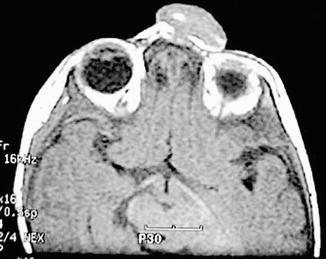

Fig. 6.5
Postnatal T1-weighted MRI confirming a mass protruding at the level of the left nasal ala: note the isointensity of the lesion compared to gray matter
Regarding management, endoscopic surgery is considered appropriate for the removal of an intranasal glioma without intracranial extension [14]. Radical excision is essential because nasal glioma or nasal glial heterotopia may recur following incomplete surgical resection [15].
6.2 Vascular Anomalies
Vascular anomalies can be divided into two main groups: vascular tumors and vascular malformations.
6.2.1 Embryology and Pathology
In general, vascular tumors demonstrate endothelial cell proliferation in contrast to vascular malformations, in which mitotic activity is typically very low or absent. However, exceptions occur. For example, in the involuting phase of infantile hemangioma, cellular proliferation is extremely reduced. On the other hand, capillary expansion may be particularly active in arteriovenous malformations [16].
Congenital nonprogressive hemangioma includes two clinical entities according to their postnatal progression: rapidly involuting congenital hemangiomas (RICHs) and noninvoluting congenital hemangiomas (NICHs). RICHs tend to fully regress after birth leaving a soft tissue defect with local loss of dermal and subcutaneous tissue. Histologically, they appear as NICHs, but with smaller lobules. NICHs are composed of large lobules of small, thin-walled vessels with curved lumina and a large, often stellate, central vessel. Endothelial cells may be hobnailed with eosinophilic cytoplasmic inclusions. Hemosiderin or extramedullary hematopoiesis may also be observed [17].
Histogenesis of congenital hemangiomas is controversial; few theories have been suggested. One theory proposes that placental trophoblasts and endothelial progenitor cells proliferate in an environment of cytokines and estrogen. Indeed, angiopoietin growth factors and cytokines (VEGF, b-FGF, IGF, and matrix metalloproteinase-9) increase during the progression phase of hemangioma formation and subsequently decrease during the involution process. Moreover, in some familial hemangiomas, mutations have been found in growth factor receptors (FGFR4, PDGFRB, VEGFR2, and Flt-4) [18–21].
Vascular malformations are considered to be the result of defective morphogenesis. They include capillary malformations (CMs), venous malformation (VMs), lymphatic malformations (LMs), and arteriovenous malformations (AVMs). CMs are the most common and usually arise along the trigeminal nerve. Light microscopy reveals dilation of venules and capillaries within the papillary and reticular dermis, sometimes extending to the superficial subcutis (Figs. 6.6–6.13).
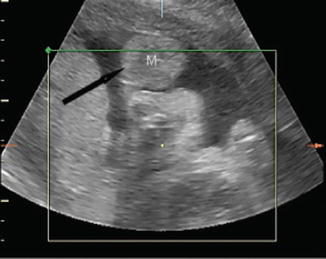
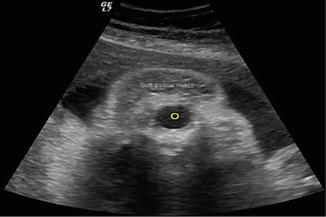
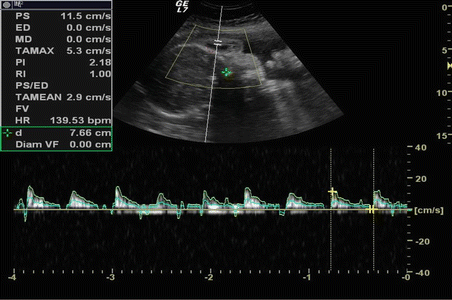

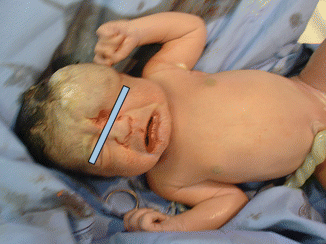

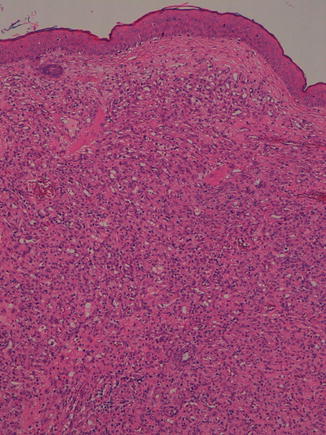
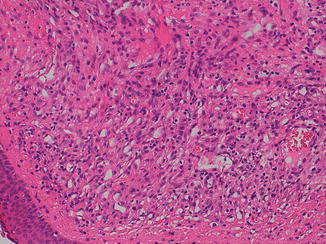

Fig. 6.6
Three-dimensional ultrasound in multiplanar mode performed at 34 weeks of gestation: a superficial mass involving the left side of the fetal profile is visible (black arrow) (M mass)

Fig. 6.7
Two-dimensional ultrasound: note the soft tissue mass located superiorly to the left fetal orbit

Fig. 6.8
Doppler ultrasound waveform analysis demonstrating a low velocity arterial flow (peak systolic velocity: 11.5 cm/s) pattern at the level of the peripheral vascular branching

Fig. 6.9
Fetal T2-weighted MRI in coronal planes (a, b) confirming the presence of a heterogeneous, hyperintense superficial lesion extending from the left temporal region and involving the conic portion of the ipsilateral orbit. Axial plane (c) demonstrating integrity of the skin overlying the lesion which extends from the left temporal region to the left fetal orbit and abutting the most medial portion of the contralateral orbit

Fig. 6.10
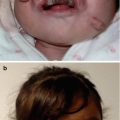
Infant with capillary malformation

Fig. 6.11
Intraoperative finding at the time of excision of capillary malformation

Fig. 6.12
Lobular capillary hemangioma is composed of small vascular channels separated in lobules by fibrous connective tissue (hematoxylin and eosin, 4 HPF). (Courtesy of Prof. N. Sebire)

Fig. 6.13
The capillaries are lined by plump endothelial cells supported by pericytic elements (hematoxylin and eosin, 20 HPF). (Courtesy of Prof. N. Sebire)
6.2.2 Embryology and Pathology
VMs are soft tissue lesions that may be multifocal or segmental and may occur on mucosal surfaces. They are composed of variably sized, thin-walled, dilated veins with scant mural smooth muscle. Due to the slow velocity of blood flow within them, thrombosis is frequent with consequent organization and recanalization, inducing endothelial hyperplasia known as Masson vegetant intravascular hemangioendothelioma.
Venous maldevelopment seems to be caused by abnormalities in the TIE-2 receptor. Mutations in the TIE-2/TEK gene on chromosome 9p21–22 lead to increased autophosphorylation of the receptor, resulting in alterations in endothelial migration and vascular growth. Activating mutations of TIE-2/TEK have been identified in some forms of hereditary VMs.
LMs may be macrocystic or microcystic. Macrocystic variants include large interconnected lymphatic vessels lined by endothelium containing proteinaceous fluid, some lymphocytes, macrophages, and erythrocytes. Some vessels may have valves and an incomplete smooth muscular wall. Mural lymphoid aggregates are frequently observed [1]. Cystic hygromas, typically located in the neck and axillae, show marked dilatation of the lymphatic spaces.
Microcystic LMs are made of smaller lymphatic vessels that may infiltrate and expand into adjacent tissues. In superficial lesions, they can form lymph-filled vesicles due to protrusion and expansion of dermal papillae.
AVMs lack a normal capillary bed between arteries and veins. Histologically, they appear as a conglomerate of arterioles, venules, and capillaries that are haphazardly connected within a densely fibrous or fibromyxomatous dermis. Larger caliber arteries and thick-walled veins are also present. Veins are often characterized by adventitial fibrosis and irregular intimal hyperplasia [22].
6.3 Tumors of the Oral Cavity and Oropharynx Teratomas
6.3.1 Classification
Teratomas of the oral cavity can be classified according to the site of origin as episphenoid, epipalatine or epignathus.
6.3.2 Embryology and Pathology
Histologically, these tumors can be divided into (1) dermoids or hairy polyps, composed of mesodermal and ectodermal elements; (2) teratoids and teratomas, which include all three germ layer components with scarce or intermediate differentiation; and (3) epignathus, which also comprises fetiform structures. The term fetus in fetu should be reserved to tumors in which it is possible to recognize axial differentiation of limbs and organs [23]. This may be the result of an arrested development of a conjoined twin from the third week of gestation. However, epignathus is commonly thought to arise from pluripotent cells in the region of Rathke’s pouch, in particular the craniopharyngeal canal, within the sphenoid bone where during early gestation, the oropharyngeal membrane, Rathke’s pouch, and the notochord are closely related [24].
Regarding the formation of congenital masses (either teratomas or hamartomas), the coexistence of collapse and expansion of the cephalic midline is observed in cases of cephalic teratomas (CTS) and holoprosencephaly diencephalic hamartoma association [25]. Therefore, midline CTS and hamartomas may be interpreted as clonal expansion of cells that escape differentiation due to the collapse (holoprosencephaly) or splitting at the midline (craniofacial duplication) of the normal process of lateralization starting during gastrulation at the cephalic pole. Nasopharyngeal teratoma predominantly develops during splitting phase of gastrulation, while diencephalic hamartoma results from with defective cleavage of the prosencephalon [26].
Dermoid or hairy polyps are polypoidal masses covered by the skin and composed of mature elements derived from ectoderm (skin and neural tissue) and mesoderm (adipose tissue, muscle, and cartilage) [27] (Figs. 6.14 and 6.15).
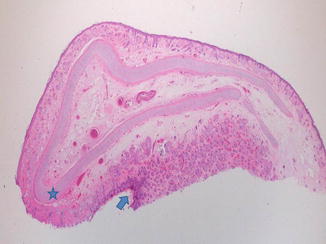
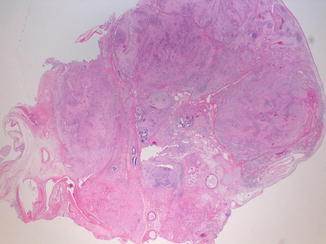

Fig. 6.14
Hairy polyp is a congenital malformation arising from the oropharynx or nasopharynx. The lesion is polypoid and layered by epidermis (blue star), hair follicles, and sebaceous glands (blue arrow). Cartilage may be found within the polyp core (hematoxylin and eosin, 2 HPF). (Courtesy of Prof. N. Sebire)

Fig. 6.15
Grossly, the teratoma is encapsulated and lobulated. Histologically, the tumor is composed of mature or immature tissues from the three embryonic germ cell layers: ectoderm, endoderm, and mesoderm (hematoxylin and eosin, 2 HPF). (Courtesy of Prof. N. Sebire)
Teratoids include the three germ layers with poor differentiation and organization [28]. However, teratoid cysts of the floor of the mouth are complex structures with an outer layer containing epithelial and non-epithelial components such as bone, muscle, blood vessels, collagen fibers, and respiratory and gastrointestinal tissue [29].
Teratomas are composed of a variable admixture of skin, skin appendages, smooth muscle, cartilage, and respiratory and gastrointestinal epithelium. Neural tissue may be present and is usually immature (“immature teratoma”) including neuroblasts, ependymal rosettes, and retinal anlage [30]. Teratomas are usually benign (Fig. 6.16).
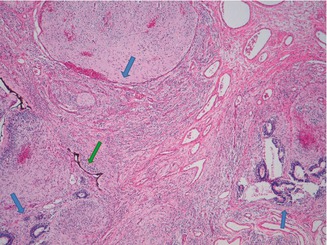

Fig. 6.16
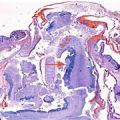
In the teratoma tumors, the most common finding is neural and glial tissue, usually organized in islands (blue star), and rosette-like formations of immature ependymal epithelium (blue arrow). Sometimes, it is possible to observe retinal anlage epithelium (green arrow) (hematoxylin and eosin, 10 HPF). (Courtesy of Prof. N. Sebire)
Epignathus is a congenital oropharyngeal teratoma originating from the base of the skull and usually involves the posterior nasopharynx, hard palate, or sphenoid bone.
6.3.3 Embryology and Pathology
Epignathus has various etiologies, including chromosomal abnormalities (such as trisomy 13 [33]), ring X-chromosome mosaicism with inactive ring X-chromosome [34, 35], gonosomal pentasomy 49, XXXXY karyotype [36], gene mutations (e.g., HLXB9) [37], or abnormalities in early embryonic development [38]. Epignathus may be a part of genetic syndromes, such as Aicardi syndrome (agenesis of the corpus callosum, infantile spasm, and ocular anomalies) [39] and Pierre Robin syndrome [40]. Although usually benign, 10 cases of malignant teratoma have been reported in the medical literature [41–45].
6.3.4 Diagnosis
Two-dimensional (2D) ultrasound has accurately identified these lesions, even at the early gestational ages of 15–17 weeks [46, 47]. However, the vast majority of tumors are usually detected in the late second and third trimesters [1], which suggests that the tumor can develop later in pregnancy [31, 48–55].
The ultrasonographic findings of epignathus is characterized by the presence of a solid or mixed cystic/solid mass and calcifications within the mass, which are virtually pathognomonic of teratoma. Color Doppler ultrasound of the solid portions shows extensive vascularization [56] (Fig. 6.17).
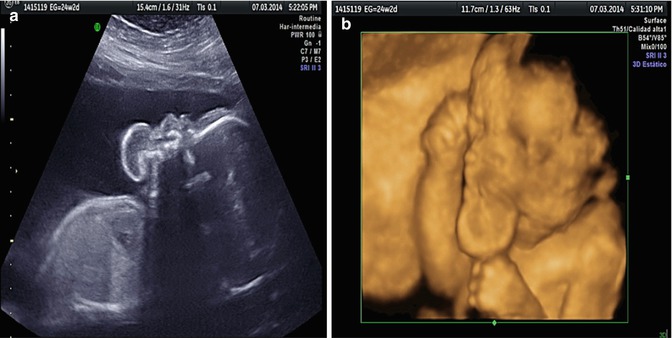

Fig. 6.17
Two-dimensional ultrasound performed at 24w2d showing, in sagittal plane, a heterogeneous mass protruding from the fetal mouth. Polyhydramnios was an associated finding (a). Three-dimensional ultrasound with surface rendering confirming the presence of an oral mass (b). (Courtesy of Prof. W. Sepulveda)
The neck is often hyperextended and polyhydramnios is usually associated. The importance of applying three-dimensional ultrasonography with rendering mode to demonstrate the spatial relationships of the tumor with the oral cavity and to provide correlations between the ultrasound images and the postnatal anatomic findings has been recently stressed [57].
Magnetic resonance (MR) imaging can be particularly useful in identifying bidirectional lesions with intracranial extension; these masses are invariably associated with a poor prognosis [58, 59] (Figs. 6.18–6.20).
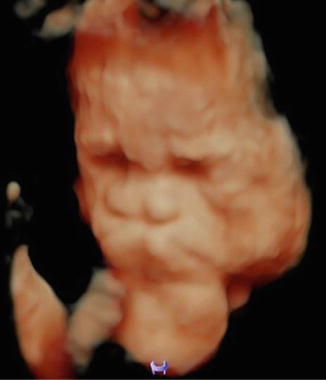
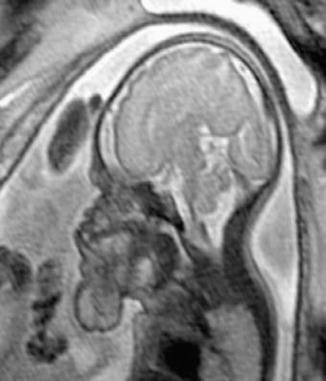
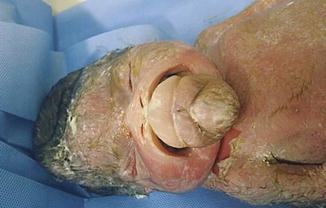

Fig. 6.18
Three-dimensional ultrasound in a second trimester fetus with epignathus using HDLive™ mode. (Courtesy of Prof. W. Sepulveda)

Fig. 6.19
T2-weighted fetal MRI. In the sagittal plane, a bulky mass is seen that is in direct communication with the oral cavity but the upper airway remains unobstructed (Courtesy of Prof. W. Sepulveda)

Fig. 6.20
Postmortem finding in a case of epignathus. Note the associated hypertelorism (Courtesy of Prof. W. Sepulveda)
MR imaging also delineates the level and extent of airway obstruction and can quantify fetal lung volumes. MR imaging can be used with either single-shot spin echo (SSSE) or half-Fourier single-shot turbo spin echo (HASTE) techniques. The latter technology enables rapid imaging in steady-state procession (FISP) to be performed in approximately 20 s and provides superior contrast resolution compared to the T2-weighted HASTE technique [30]. MR imaging is critical to accurate diagnosis, especially in confirming whether the CNS is involved, and for optimal delivery planning for fetuses in which an EXIT (ex utero intrapartum treatment) procedure may be indicated [60].
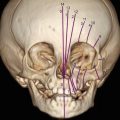
Very recently, Heron et al. have demonstrated the utility of integrating 3D ultrasound with MRI volume data to reconstruct a physical model of the lesion and to evaluate for the possibility of fetal upper airway obstruction. To construct the physical model from 3D ultrasound and MRI, the first step is to create a virtual 3D model. All the images obtained through 3D ultrasound and MRI are exported to a workstation in DICOM format. The 3D structure of the fetus is reconstructed by generating its surface using software with the capacity to convert the images obtained into numerical models. These reconstructed images are then exported using STL (standard triangular language) and converted to the file extension 3D polygonal modeling software. The 3D model is converted back to the “STL” extension and exported to the Mimics software, which correlates the shapes and outlines observed from 3D ultrasound. Finally, to determine the physical model of the fetus, ultraviolet spot laser beams are guided through a reservoir of photosensitive resin to model the fetal shape based on the data stored in the 3D geometry software, sliced into transverse planes of predefined thickness of 0.1–0.2 mm [61] (Fig. 6.21).
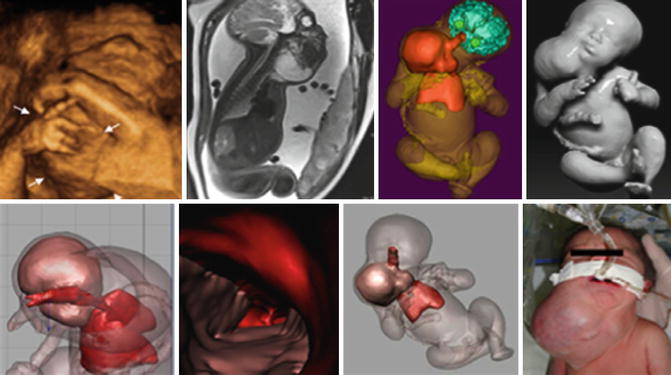

Fig. 6.21
Giant latero-cervical teratoma. (a) 3D ultrasound demonstrates the mass (arrows) extending from the neck region (b) Fetal T2-weighted MRI confirmed heterogeneous hyperintense as well as hypointense signals within the mass (c–g) Physical models obtained from fetal MRI volumes with reconstruction of the airway and larynx, including demonstration of the anatomic relationship between the teratoma and the airway (h) EXIT procedure following delivery by Cesarean section (Courtesy of Dr. W. Heron)
6.3.5 Associated Anomalies
Epignathus should be differentiated from cervical teratoma, cystic hygroma, congenital goiter, branchial cyst, parotid tumor, carcinoma of the thyroid [43], and other rare tumors involving the soft tissue such as hemangioma, fibromatosis, fibrosarcoma, rhabdomyosarcoma [62], cervical neuroblastoma, anterior encephalomyelocele [63, 64], and cavernous lymphangioma of the face and neck, which is a rare variant of cystic hygroma colli characterized by the involvement of deep subcutaneous tissues and muscular septa [65].
Epignathus is often associated with a markedly elevated α-fetoprotein level on second trimester aneuploidy screening [32]. In addition, epignathus is frequently associated with polyhydramnios (in approximately 30 % of cases) due to obstruction of the fetal mouth and impaired fetal swallowing of amniotic fluid [48, 60] that may cause severe respiratory compromise at delivery.
6.3.6 Prognosis
Although usually benign, 10 cases of malignant teratoma have been reported in the medical literature [31, 45–48]. Prognosis depends on size and location of the tumor, rate of growth, associated polyhydramnios, and degree of intracranial spread [66].
Poor prognostic indicators on prenatal sonography include tumors of large size (>5 cm), polyhydramnios, and hydrops fetalis [39, 64, 67, 68].
In cases of giant epignathus and/or cervical teratoma, obstruction of circulation may also lead to high-output cardiac failure, with subsequent development of polyhydramnios and nonimmune hydrops fetalis, a finding that is strongly associated with intrauterine fetal demise [30]. In such cases, the perinatal mortality rate approaches 100 % if immediate critical neonatal intervention and prompt resection of the tumor is not performed [69].
6.3.7 Recurrence Risk
Congenital epignathus is not known to be associated with an increased risk of recurrence in subsequent pregnancies.
6.4 Tumors of the Tongue and Gingiva
These tumors include congenital epulis, congenital lingual dermoid cyst, oral foregut cyst, congenital ranula, teratoid cyst of the tongue, and teratoma of the tongue.
Epulis is a Greek term that means “swelling on the gingiva.” It is a rare benign tumor that usually arises from the mucosa of the anterior part of the maxillary alveolar ridge, but may be located in the mandible, maxilla, or tongue. It may be a single mass or occur as multiple lesions [70, 71].
Epulis has an estimated incidence of 1:7000 newborns with a significant female preponderance; it occurs 8–10 times more frequently in females (compared to males) [70, 71]. This discordance may be suggestive of a hormonal component to its development.
6.4.1 Embryology and Pathology
The etiology of congenital epulis remains uncertain. The tumor is also postulated to originate from undifferentiated mesenchymal cells, fibroblasts, myofibroblasts, histiocytes, pericytes, Schwann cells, or odontogenic epithelial cells. Several immunohistochemical studies also support a mesenchymal origin [72, 73].
Histologically, epulis is a well-defined lesion composed of polygonal cells with a granular, eosinophilic cytoplasm. Cells are of medium to large size, and nuclei are eccentric with small nucleoli. Atypical nuclei may be seen, but the overall appearance is bland. The lesion is covered by a thin stratified squamous epithelium with a variable degree of keratinization and absence of rete ridges. Epulis variants have been described such as increased fibrosis and spindle cell features. Staghorn-like vascular channels, occasional nests or cords of odontogenic epithelium, and lymphohistiocytic infiltration have also been reported. Epulis immunoreactivity is for vimentin and neuron-specific enolase (NSE).
Histogenesis of this entity is controversial and mainly unknown. Many hypotheses have been considered, including a form of odontogenic dysgenesis, which is maldevelopment of the tooth germ. However, the presence of occasional ameloblast-like cells among the granular cells is not sufficient evidence because epithelial residues can be found in all gingival tissues. Other theories consider possible odontogenic, fibroblastic, histiocytic, myogenic, and neurogenic origins. Among the numerous hypotheses, the most widely recognized is a degenerative process of undifferentiated mesenchymal cells [74–76] (Figs. 6.22).
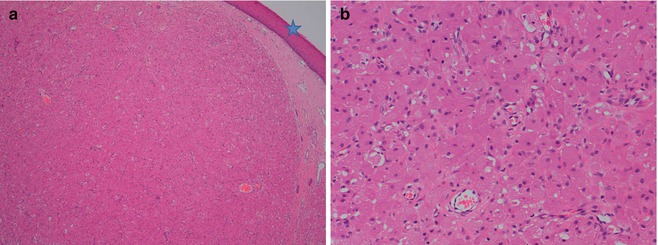

Fig. 6.22
(a) The congenital epulis is usually a submucosal lesion with overlying intact epithelium (blue star). Cells grow in a typical sheet-like pattern surrounded by fibrovascular septa (hematoxylin and eosin, 4 HPF) (b) The epulis is composed of large, granular cells with abundant eosinophilic cytoplasm. The nuclei are round and basophilic (hematoxylin and eosin, 40 HPF). (Courtesy of Prof. N. Sebire)
Ultrastructural studies of epulis demonstrate the presence of many autophagosomes containing collagen precursors, suggesting the tumor cells represent early mesodermal cells that express pericytic and myofibroblastic features that undergo cytoplasmic autophagocytosis [77].
Congenital epulis is usually seen at birth and has a predilection for the maxillary alveolar process, lateral to the midline in the region of the primary canine and lateral incisor. Less frequently, it has been reported to occur on the mandibular alveolus and tongue, and in one case with the involvement of the alveolar ridge as well as the tongue [78]. Typically, a single tumor is present (90 %), ranging in size from several millimeters to several centimeters [79].
6.4.2 Diagnosis
Since its first description in 1871 in Germany as “congenital epulis” by Neumann [78], over 200 cases of this rare lesion have been reported [70].
Congenital epulis appears on ultrasound as a well-defined, round, hypoechoic mass protruding from the mouth and with a branching pattern of feeder vessels [79–81] (Fig. 6.23). MRI features of epulis are characterized by a hypointense T2-weighted signal (Fig. 6.24).
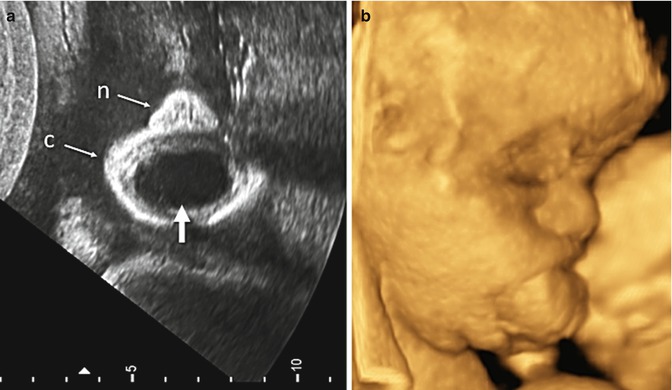
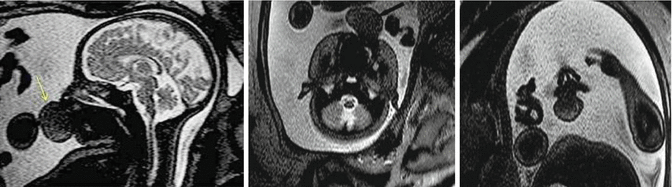

Fig. 6.23
Prenatal imaging of a fetus at 31 weeks 1 day with epulus. (a) Two-dimensional ultrasound demonstrated persistent opening of the mouth due to a protruding cystic mass (arrow). n, nose, c, chin. (b) Three-dimensional surface-rendered ultrasound clearly depicting the protruding mass (Courtesy of Prof. W. Sepulveda)

Fig. 6.24
Fetal MRI with hypointense T2-weighted signal in a fetus at 35 weeks of gestation with epulis (yellow arrow) in sagittal, axial, and coronal plane, respectively. (Courtesy of Dr. A. Rossi)
Accelerated growth during the third trimester has been reported in some cases; thus, periodic ultrasonographic evaluation is recommended. Repeat exams should focus on changes in the size of the mass and attempt to evaluate fetal breathing and swallowing, including monitoring for increased amniotic fluid volume or absence of a full stomach that would suggest impairment of fetal swallowing [82–84].
6.4.3 Associated Anomalies
There are usually no associated dental abnormalities or congenital malformations, except for occasional reports of a hypoplastic or absent tooth and the possibility of mild midface hypoplasia [70]. Congenital epulis has been reported in infants with polydactyly, goiter, triple X syndrome, maxillary hypoplasia, neurofibromatosis, and polyhydramnios [85].
6.4.4 Prognosis
6.4.5 Management
Arising from the mucosa of the gingiva and protruding out of the infant’s mouth, a very large epulus can potentially obstruct the airway so early surgical resection is recommended. An EXIT procedure is the ideal delivery strategy for fetuses with a prenatally diagnosed epulis that is sufficiently large to pose concern for potential airway obstruction at birth. Laje et al. [87] performed a retrospective review on four newborns delivered by an EXIT procedure, two of whom were prenatally diagnosed with congenital epulis and two with epignathus. The study analyzed multiple parameters including maternal time under anesthesia, maternal operative time, maternal blood loss, maternal hospital stay, and hysterotomy-to-cord clamp time. The airway was successfully accessed via direct laryngoscopy before the umbilical cord was clamped. Some authors indicate that 60 min is the limit for placental support while performing the EXIT procedure [88].
6.4.6 Recurrence Risk
Congenital epulis is not known to be associated with an increased risk of recurrence in subsequent pregnancies.
Congenital lingual dermoid cysts are squamous epithelial-lined cavities with variable numbers of skin appendages in the capsule and are rare entities in the head and neck. Lingual dermoid cysts are most commonly present in early childhood or adolescence and are located in the anterior two-thirds of the tongue. Treatment of these lesions consists of complete surgical excision; however, midline sagittal glossotomy incision using the CO2 laser has been proposed as it offers surgical precision, superior hemostasis and wound healing, and minimal postoperative edema [89].
Oral foregut cyst is a rare congenital choristoma lined by respiratory and/or gastrointestinal epithelium that should also be considered in the differential diagnosis of an oral tumor. The exact etiology has not been fully identified, but it is thought to arise from misplaced primitive foregut. This lesion initially develops without symptoms but may then lead to difficulty with swallowing and speech depending on its size. Thus, the first choice of treatment is surgical excision. Surgeons should include the oral foregut cyst in the differential diagnosis for ranula, dermoid cyst, thyroglossal duct cyst, and lymphangioma in cases of pediatric head and neck lesions [90]. MR imaging demonstrates a low attenuated T1-weighted signal in the ventral tongue and a high attenuated T2-weighted signal, consistent with a fluid accumulation lesion [91].
Ranulas are mucoid retention cysts, or mucoceles, arising from the sublingual gland or its ductal system secondary to obstruction. A ranula may rarely extend inferomedially into the inferior portion of the tongue resulting in a cystic tongue lesion [92]. Ranulas are extremely rare tumors without a known incidence.
6.4.7 Embryology and Pathology
Ranulas may remain in the floor of the mouth or extend below the mylohyoid sling (“plunging” or “diving” ranula). Cervical ranulas are those that have paracervical extension and plunging ranulas are those that extend toward the superior airway. Another possibility is that a lingual ranula may arise from obstruction of a minor salivary gland or embryologically ectopic salivary tissue [93–95].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree