Chapter 86 Evaluation of the organs in the gastrointestinal tract and within the peritoneal cavity is a standard part of the full prenatal ultrasound scan for anatomy screening.1 In circumstances when ultrasound cannot accurately determine the extent or nature of an abnormality, fetal magnetic resonance imaging (MRI) can be useful. Sequence selection includes standard single-shot fast spin echo (SSFSE) T2-weighted images at 3- to 5-mm slice thickness and steady-state free-precession imaging (SSFP) at overlapping intervals. Echo-time lengths range from 80 to 250 ms; longer lengths are advantageous for delineation of cystic abnormalities. T1-weighted fast gradient echo (GRE) sequences may be useful in the fetal abdomen, specifically for identification of the liver, which is mildly hyperintense, and very bright signal meconium.2 Imaging modalities using ionizing radiation have no role in the evaluation of a fetus with a gastrointestinal (GI) tract abnormality, which can be categorized into one of several general categories: obstructions, ventral wall defects, masses and solid organ abnormalities, echogenic bowel, and peritoneal abnormalities. Etiology: Interruption of the fetal GI tract can occur at any point from the esophagus to the anus, and the etiology for the loss of continuity is not the same at each site. The cause of esophageal atresia (EA) with or without a fistula (communicating between the trachea and the esophagus distal to the atretic level) is not known but is believed to be a result of failure of the tracheal bud to develop normally from the primitive foregut (see Chapter 97).3,4 EA occurs in approximately 1 in 4000 live births and can be associated with a number of additional abnormalities in the fetus. Most commonly these abnormalities include those comprising the VACTERL complex (vertebral, anal, cardiac, tracheoesophageal, renal, and limb anomalies) and those seen in Down syndrome, such as common atrioventricular canal, absent or hypoplastic nasal bone, and nuchal thickening. EA occurs most commonly with a distal fistula (84%).5 Gastric atresia is the rarest atresia and is almost always pyloric with a distended proximal stomach. It likely results from vascular impairment during fetal development and is associated with epidermolysis bullosa.6,7 In contrast to pyloric atresia, duodenal atresia (DA) is relatively common, and along with EA is associated with Down syndrome. The etiology of DA may be exceptional in that it is believed to be due to dysfunction in embryonic recannulation of this segment of the proximal small bowel (see Chapter 103).8 Atresias distal to the duodenum are thought to be the result of ischemia or other focal insult to the bowel and may be multiple. Thus it is prudent not to assume that the most proximal obstruction is the only site. Small bowel atresias are the most common cause of fetal and neonatal bowel obstructions, with colonic segments affected much more rarely (in 10% of all atresias).9,10 Although most atresias are sporadic, some populations are prone to multiple atresia syndromes.11,12 Meconium ileus in persons with cystic fibrosis also can have small bowel atresia, and colonic atresia can be seen in persons with Hirschsprung disease.13,14 Anorectal atresia/malformation deserves special consideration because it often is seen in combination with other malformations, such as VACTERL, caudal regression, cloacal malformation, or the Currarino association. Imaging of the Esophagus: Despite the likelihood of a distal fistula that might allow for flow of fluid into the gut, the most consistent clues to the diagnosis of EA on ultrasound are a persistently small-volume stomach and polyhydramnios. Distension of a proximal esophageal pouch typically is intermittent and therefore unreliably witnessed either on ultrasound or MRI, although in the rare cases where it is vital to make a prenatal diagnosis, serial midline sagittal MRI with cine SSFP sequences may prove useful (Fig. 86-1).15 Figure 86-1 Esophageal atresia. Imaging of the Stomach: A lack of visualization of the stomach is most likely EA with or without a distal fistula. Other considerations in the differential diagnosis of a small stomach at fetal imaging include congenital microgastria; oligohydramnios/anhydramnios, with a lack of fluid to swallow and distend the stomach; diminished swallowing function (e.g., in arthrogryposis, with little to no fetal movement); or obstruction to normal swallowing, including from increased intrathoracic pressure (Fig. 86-2). A markedly distended stomach, on the other hand, might raise concern for outlet obstruction, as with pyloric atresia. Gastric volvulus rarely has been reported in utero and is more of a risk when the stomach is not in its normal location.16 A right-sided stomach in situs ambiguous or inversus should be evident as long as one is careful to establish the orientation of the fetus to the mother during the examination (Fig. 86-3). Figure 86-2 A small collapsed stomach (arrow) in a fetus with hydrops, hypotonia, and limited swallowing. Imaging of the Duodenum: DA appears on imaging as the classic fluid-filled double bubble of dilated stomach and duodenal bulb (Fig. 86-4). Duodenal stenosis sometimes can be distinguished by hyperperistalsis of the dilated proximal duodenum, whereas in DA, no peristalsis may exist at all. DA is associated with malrotation of the bowel in approximately 29% of cases.8 Other causes for proximal obstruction include an adhesive Ladd band in a patient with primary midgut malrotation, or annular pancreas. As with other obstructions to the GI tract above the level of the distal jejunum, a fetus with duodenal obstruction typically has polyhydramnios; the more proximal the atresia, the earlier and the more frequently an abnormally large amniotic fluid volume tends to present.17 Imaging of the Small Bowel: The small bowel is considered to be dilated if it is larger than 7 mm; the colon is usually up to 15 mm in the third trimester, while the normal rectum, which should be the largest segment, can be larger18–20 (Fig. 86-5). In proximal jejunal atresia, more loops will be dilated than just the double bubble seen with DA (“triple bubble” in very proximal jejunal atresia) (Fig. 86-6); distal jejunal atresia and ileal atresia, on the other hand, may be more difficult to distinguish prospectively because multiple dilated, fluid-filled loops are seen in both conditions, and typically a normal amniotic fluid volume is present. Other considerations for a distal bowel obstruction parallel those in newborn imaging and include meconium ileus in a fetus with cystic fibrosis; midgut volvulus; volvulus around a residual of the omphalomesenteric duct; and total colonic aganglionosis (Hirschsprung disease). One clue to the diagnosis of meconium ileus might be the presence of hyperechoic debris filling distended loops near the point of obstruction in the distal small bowel on ultrasound; MRI shows that the internal contents are hyperintense on T1-weighted images but intermediate on T2 imaging, consistent with meconium. One would expect low-signal T2 contents with the more simple intraluminal fluid in the fetus with ileal atresia.21–23 Vigorous hyperperistalsis of the bowel is indicative of obstruction (Fig. 86-7 and Video 86-1). Cases of in utero volvulus, although uncommon, have been prospectively identified when the dilated ischemic segments are thick walled, nonperistaltic, and have internal echogenicity from hemorrhage.24 Figure 86-5 Ileal atresia. Figure 86-6 Proximal jejunal atresia. Figure 86-7 Duodenal obstruction due to Ladd’s band in malrotation. Imaging of the Colon: Colonic atresias are difficult to diagnose specifically unless the obstructed segment can be identified with certainty in the expected location of the colon, or interhaustral notches can be separated from valvulae conniventes on ultrasound. Meconium typically is seen in the rectum by 20 weeks’ gestation, in the left colon by 24 weeks’ gestation, and in the right colon by 31 weeks’ gestation, but this pattern may be altered by the pathology that is present.25 On FSE T2-weighted sequences, meconium has a very low signal, whereas it shows a high signal on T1 weighting. In general, when dilated loops are discovered, ultrasound is sufficient to diagnose the level of obstruction as proximal versus distal and to generate a focused differential diagnosis. Fetal MRI is reserved for complex cases or for those in which other abnormalities are suspected and might be confirmed by additional imaging.26 The advantage of MRI is in its larger field of view and reproducible multiplanar capability, showing the anatomy relative to other landmarks.27 This advantage usually is demonstrated well with single-shot T2-weighted sequences in multiple planes, whereas the addition of GRE T1-weighted sequences to show the distribution of high-signal meconium can provide useful additional information (Fig. 86-8). It is crucial to understand that meconium is not exclusively restricted to the colon, can normally be seen in the distal ileum, and may be in dilated loops proximal to distal obstructions (Fig. 86-9).28 Box 86-1 lists causes of nonvisualization of meconium in the colon. Figure 86-8 Meconium. Figure 86-9 Meconium ileus. Although one might anticipate that bowel loops would be dilated in cases of anorectal atresia, such dilation is uncommon, and the diagnosis often is made on the basis of a high level of suspicion because of associated features.29 On ultrasound and MRI, the diagnosis may be suspected if the characteristic hypoechoic configuration of the anal musculature (Fig. 86-10) is absent. Also, a cord or ridge of abnormal fibrous tissue at the perineum may be imaged by ultrasound or MRI (Fig. 86-11). If a fistula between the colon and the urinary tract allows mixing of meconium and urine, intraluminal precipitant calcifications may be detected (Fig. 86-12). Figure 86-11 Typical configuration of the perineal cord (arrowheads) that may be seen from the genitalia toward the level of anal atresia. Figure 86-12 Cloacal malformation. Congenital diarrhea syndromes have been reported as showing dilation of the bowel to the rectum but without meconium signal on T1-weighted imaging, reflecting the loss of normal meconium.30 Therefore lack of colonic meconium is not exclusive to mechanical small bowel obstructions. Differential considerations include secretory sodium or chloride diarrheas, megacystis-microcolon-hypoperistalsis syndrome, pseudoobstruction, and total colonic aganglionosis (Hirschsprung disease).25,28,30,31 Treatment and Follow-up: At this time, no criteria have been established for intervention in the fetus based on bowel obstruction unrelated to ventral wall defects, aside from amnioreduction for polyhydramnios. Selected patients may have amniocentesis to evaluate for the possibility of chromosomal abnormalities or cystic fibrosis. Follow-up is performed with ultrasonography, with a plan to have the newborn evaluated at birth by the appropriate pediatric specialists. Etiology: Normal herniation of the midgut into the proximal umbilical cord occurs in the eighth gestational week, and after undergoing 270 degrees of rotation, the midgut returns to the abdominal cavity for fixation by the end of the twelfth week. Visualization of either the liver or bowel in the umbilical cord after 12 weeks is abnormal. Ventral wall defects include all abnormalities in which extrusion of internal anatomic structures to the outside of the fetal abdomen and/or pelvis occurs. In general, these abnormalities are thought to occur from a failure of the normal infolding and fusion of the embryonic disc.32 If craniad infolding fails, the resulting defect would be to the abdominal wall and lower chest, resulting in findings in the spectrum of the pentalogy of Cantrell (omphalocele, sternal defect, ventral mediastinal diaphragmatic hernia, pericardial defect, and ectopia cordis) (Fig. 86-13). Lateral fold defects lead to an omphalocele, which is the most common isolated ventral wall defect and is contained by a membrane (parietal peritoneum and amnion) unless ruptured (Fig. 86-14).33 Caudal fold failure results in bladder or cloacal exstrophy, which primarily affect the genitourinary tract. In bladder exstrophy, the ventral wall of the bladder, inferior rectus musculature, and skin are absent, and the residual dorsal wall of the bladder is continuous with the abdominal wall. Cloacal exstrophy is more complex; persistence of the infraumbilical cloacal membrane is thought to prevent normal anterior abdominal wall closure and separation of the urogenital system from the rectum, and as a result, two hemibladders are separated by bowel. Closed spinal defects, renal and genital malformations, and club feet may occur. Both bladder and cloacal exstrophy include diastasis of the pubic symphysis and may have more complex pelvic bone deformities (Fig. 86-15). OEIS complex describes the particular association of omphalocele, bladder exstrophy, imperforate anus, and spinal defect,34 but this complex is believed by many persons to be a synonym for cloacal exstrophy.35 Figure 86-13 Pentalogy of Cantrell. Figure 86-14 Omphalocele. Body stalk anomaly is a severe form of ventral wall defect that results from failure of folding along multiple planes and is not compatible with extrauterine life (Fig. 86-16).36 Figure 86-16 Limb-body wall complex. Gastroschisis is a type of ventral wall defect that may be unrelated to embryonic disc folding. It is possible that a vascular insult results in a through-and-through defect in the layers of the abdominal wall adjacent to the umbilicus, almost universally on the right, through which the bowel herniates without a covering membrane; other hypotheses have been proposed, but the cause is not yet known (Fig. 86-17).37,38 The risk of bearing a fetus with gastroschisis is reportedly increased in young primigravida women and also has been associated with the use of agents that may cause vasoconstriction, such as tobacco and salicylates.39,40
Prenatal Gastrointestinal and Hepatobiliary Imaging
Intestinal Obstructions
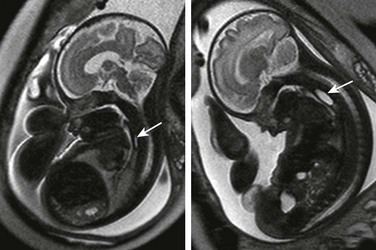
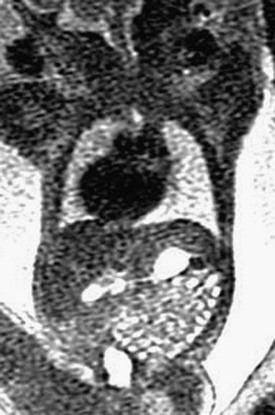
Serial imaging may show intermittent phenomena: A sagittal single-shot fast spin echo image in the neck and chest shows a small amount of fluid in the proximal esophagus that distends to a pouch on a later sequence (arrows). Cine steady-state free-precession sequences are especially useful for serial imaging because of their fast acquisition times.
Note the lack of any motion artifact in the amniotic fluid.
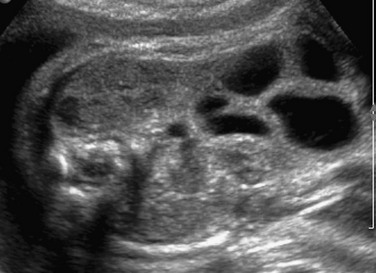
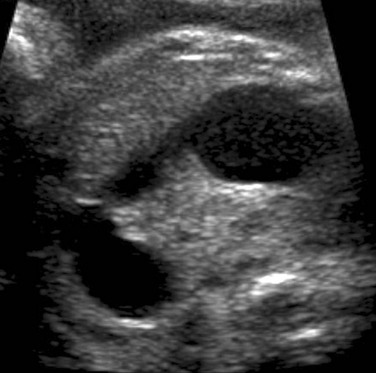
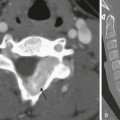
A coronal sonogram of the abdomen shows multiple dilated small bowel loops.
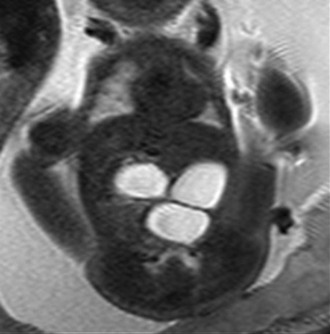
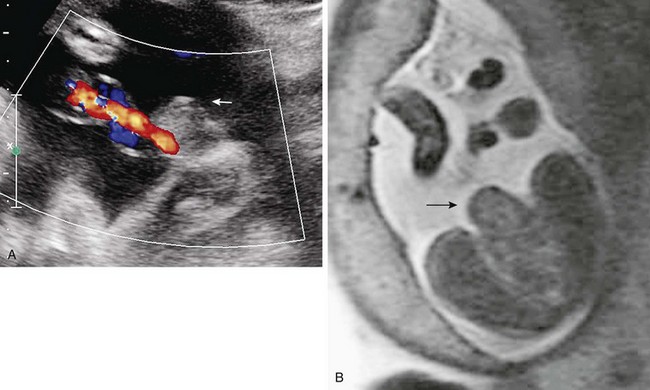
A single-shot fast spin echo coronal image with a dilated stomach, duodenal bulb, and proximal jejunum (“triple bubble”).
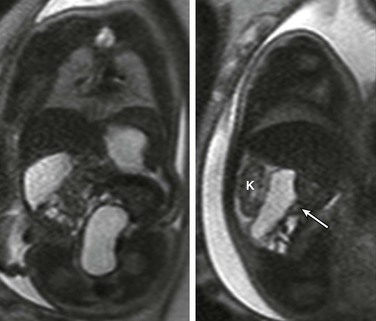
Coronal (left) and sagittal (right) single-shot fast spin echo images show the stomach in the left upper quadrant and a dilated segment of bowel in the right upper quadrant (arrow) ventral to the right kidney (K). The ultrasound clip shown in Video 86-1 is remarkable for hyperperistalsis of this segment.
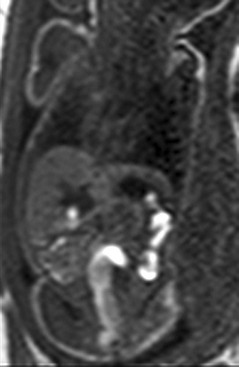
A coronal T1-weighted gradient echo image showing T1 high-signal meconium in the colon.
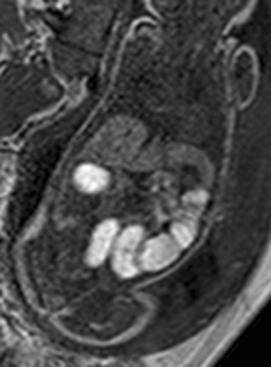
A gradient echo T1-weighted image at 32 weeks demonstrates dilated small bowel containing high-signal meconium.
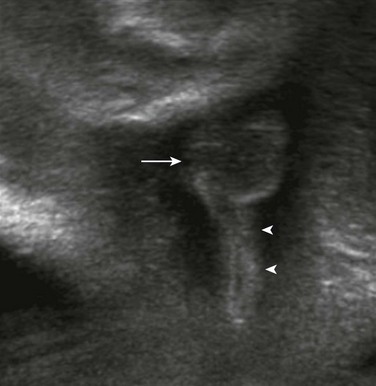
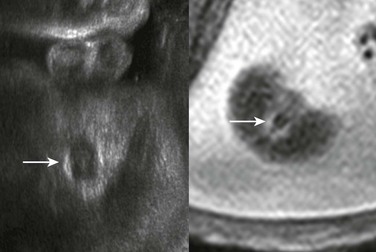
The arrow indicates the scrotum in this male fetus.
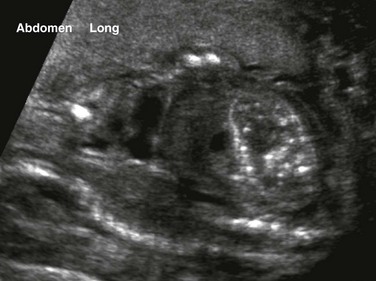
An oblique coronal ultrasound shows multiple small echogenic calcifications within dilated colon in the left abdomen, resulting from mixing of meconium and urine.
Ventral Wall Defects
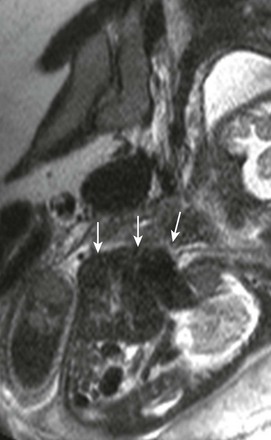
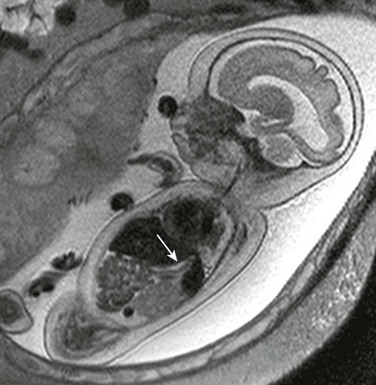
A sagittal single-shot fast spin echo image through the chest shows herniation of low-signal liver and even lower-signal heart (arrows) through a ventral wall defect.
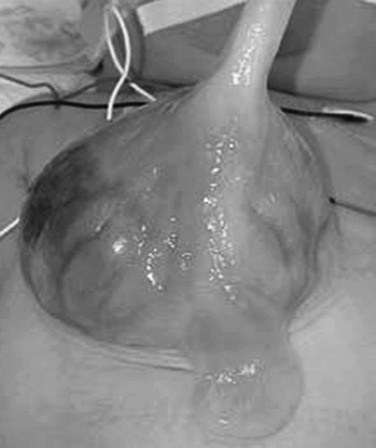
This photograph of a newborn shows a large anterior wall defect with a surrounding membrane. The umbilical cord is inserting at the apex.
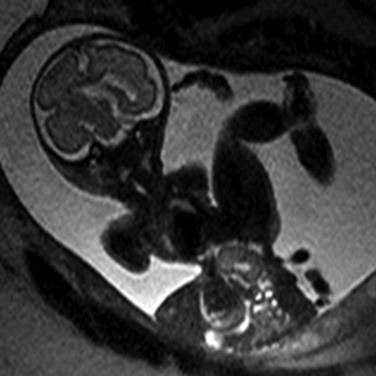
Extrusion of all abdominal contents in limb-body wall complex. Typically a very short umbilical cord and significant fetal contortion are present.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree