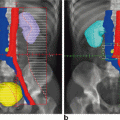
Fig. 4.1
Representative axial slices showing isodose distributions for two planes for an (a, c) IMRT and (b, d) 3D-CRT plan. The central (high dose) isodose line is more conformal with regards to the target and the peripheral (low dose) isodose lines spare more of the bowel in the IMRT versus 3D-CRT plans, respectively. IMRT intensitymodulated radiation therapy, 3D-CRT three-dimensional conformal radiation therapy. Figure reproduced with permission from Mok H, Crane C, Palmer MB, Briere TM, Delclos ME, Krishnan S, Das P. Intensity modulated radiation therapy (IMRT): differences in target volumes and improvement in clinically relevant doses to small bowel in rectal carcinoma. Radiat Oncol. 2011 Jun 8; 6: 63. Note figure legend has been modified from original. (License: http://creativecommons.org/licenses/by/4.0/)
Gynecological Malignancies
Radiation Therapy Oncology Group (RTOG) 0418 is a multiinstitutional phase II study designed to assess the feasibility of pelvic IMRT with and without concurrent chemotherapy for the treatment of 48 cervical and endometrial cancer patients in the postoperative setting. Following hysterectomy, treatment of the pelvis can expose greater amounts of small bowel that have fallen into the vacated space to radiation and lead to greater toxicity. The risk of severe injury from postoperative radiation therapy even to moderate doses is 5–15 %. Preliminary results showed equivalent 2-year disease control compared to previous trials where standard pelvic radiation was used but with a reduction in ≥ grade 2 acute GI toxicity for patients treated with IMRT [1]. Longer follow-up is needed before estimates of chronic GI toxicity may be reported. RTOG 1203 is a randomized phase III study of standard four-field radiation treatment versus IMRT pelvic radiation for postoperative treatment of endometrial and cervical cancer (TIME-C) and is currently accruing. RTOG 1203 is designed to measure patient reported acute GI toxicity through an instrument evaluating multiple components of radiation enteritis including cramping, looseness, pain, and frequency of bowel movements. Other endpoints include acute GU and hematological toxicity, QOL, and chronic GI and GU toxicity in addition to disease control. Another multicenter phase II trial reported < 30 % acute grade 2 GI toxicity in patients undergoing adjuvant IMRT for endometrial cancer [2]. In addition, Mundt et al. reported toxicity from a small single-institution study of 40 patients with gynecological malignancies who underwent IMRT. Acute grade 2 toxicity was significantly less common in the IMRT as compared to historical controls treated with 3D-CRT with 60 % versus 91 %, respectively. In addition, only 75 % of patients required little or no antidiarrheal medication as compared to 34 % of patients undergoing 3D-CRT. There were no significant differences in GU toxicity between groups. Chronic GI toxicity was also reported to be lower in the IMRT group with 11.1 % versus 50 % in the 3D-CRT group [3, 4].
Gastrointestinal Malignancies
Similar results have been shown for decreased GI toxicity in rectal and anal cancer patients undergoing IMRT. RTOG 0822 was the first multi-institutional prospective and largest phase II study of use of IMRT for preoperative concurrent chemoradiotherapy for 68 patients with rectal adenocarcinoma. There was reduced, although not statistically significant, acute ≥ grade 2 GI toxicity as compared to previous trials where standard pelvic radiation with 51 % versus 58 %, respectively. There was a high rate of contouring and planning compliance and pathological complete response rates suggesting tumor coverage was not compromised by use of IMRT [5]. In addition, Samuelian et al. conducted a retrospective single-institution review evaluating acute toxicity in 92 patients with rectal cancer who were treated with conventional 3D-CRT (66 %) or IMRT (31 %) to a median dose of 50.4 Gy using standard fractionation with concurrent chemotherapy in the neoadjuvant or adjuvant setting. Patients who received IMRT had significantly less GI toxicity, with 62 % of patients who underwent 3D-CRT experiencing ≥ grade 2 GI toxicity compared with 32 % of patients who underwent IMRT, mainly attributed to an improvement in lower GI toxicity. Among patients undergoing 3D-CRT, 48 and 30 % of patients experienced ≥ grade 2 diarrhea and enteritis, respectively, compared with 23 and 10 % of patients undergoing IMRT. There was no significant difference in hematologic or acute GU toxicity , pathological complete response rates or postoperative morbidity between groups [6].
RTOG 0529 was a phase II study of concurrent chemoradiotherapy with 5-Fluorouracil and Mitomycin-C with IMRT for the definitive treatment of 52 anal cancer patients. Two-year clinical outcomes were similar to previous trials with standard pelvic radiation. While the primary endpoint of reduced acute ≥ grade 2 GI and GU toxicity was not met, there were significant reductions in acute ≥ grade 2 hematologic (73 % vs 85 %), acute ≥ grade 3 GI (21 % vs 36 %), and acute ≥ 3 dermatological toxicity (23 % vs 49 %) with IMRT as compared to historical controls, respectively [7]. Chuong et al. compared toxicity in a single-institution retrospective review of 89 patients with anal squamous cell carcinoma treated with definitive concurrent chemoradiotherapy with either 3D-CRT (42 %) or IMRT (58 %). With a median follow-up of 26.5 months, there was significantly decreased acute ≥ grade 3 toxicity in the IMRT group with 21 % versus 60 % in the 3D-CRT group, and significantly decreased ≥ acute grade 3 skin toxicity in the IMRT group with 12 % versus 65 % in the 3D-CRT group. In addition, there was significant improvement in late ≥ grade 3 GI toxicity in the IMRT group with 6 % versus 24 % in the 3D-CRT group. There were no differences in clinical outcomes [8].
Genitourinary Malignancies
IMRT has long been established as a well-tolerated treatment modality that is associated with excellent long-term tumor control outcomes in patients with localized prostate cancer [9]. Forsythe et al. compared toxicity in a single institution retrospective review of 1571 patients with intermediate- or high-risk prostate cancer who underwent definitive combination therapy with 3D-CRT (64 %) in the earlier years or IMRT (36 %) in the later years with brachytherapy boost consisting of Pd-103 or I-125. With a median 10-year follow-up, patient-scored acute moderate urinary toxicity was similar, but there was significantly less severe acute urinary toxicity in the IMRT group with 17 % versus 44 % in the 3D-CRT group, with no difference in toxicity at 1 year. There was also significant improved urinary QOL in the IMRT group with 35 % versus 51 % in the 3D-CRT group and significantly decreased ≥ grade 2 rectal bleeding in the IMRT group with 11 % versus 7 % in the 3D-CRT group [10]. Another large single-institution study has reported on rates of improved long-term GI toxicity in patients undergoing prostate radiotherapy with IMRT versus 3D-CRT alone in the definitive setting [11].
IMRT has also been applied to the adjuvant and salvage setting for prostate cancer patients with improvements in toxicity. Alongi et al. compared acute toxicity in a single institution retrospective review of 172 patients with prostate cancer undergoing adjuvant or salvage treatment with whole pelvis radiotherapy. There was a statistically significant decrease in acute ≥ grade 2 upper GI toxicity, with 7 % versus 22 % for patients undergoing IMRT (53 %) and 3D-CRT (47 %), respectively. There was a trend towards decreased acute ≥ grade 2 GU toxicity, with 7 % versus 12 % and acute ≥ grade 2 lower GI toxicity with 3 and 9 % for patients undergoing IMRT and 3D-CRT, respectively [12]. In addition, another single institution retrospective study has reported on acute GI and GU toxicity with excellent long-term toxicity in prostate cancer patients who underwent pelvic radiotherapy with IMRT helical tomotherapy in the adjuvant or salvage setting [13].
Proton Therapy
Proton therapy is an additional modality to be considered for the treatment of prostate cancer given its precision that may allow for dose escalation with potentially less side effects as compared to conventional treatment modalities. Gray et al. compared toxicity patterns in 371 prostate cancer patients who underwent treatment with 3D-CRT (n = 123), IMRT (n = 153), or proton beam therapy (n = 95) in a prospective study through review of patient-reported outcomes at 2–3, 12, and 24 month follow-up. At first posttreatment follow-up, patients who received 3D-CRT or IMRT reported clinically meaningful decreases in bowel QOL as compared to those patients who received proton therapy. In addition, patients who received IMRT reported a clinically meaningful decrease in urinary QOL in terms of urinary irritation, obstructive symptoms, and incontinence. At 12 months, only patients who received proton therapy reported a clinically meaningful decrease in urinary irritation and obstructive symptoms. At 24 months, there were no meaningful changes in urinary QOL for all groups. At 12 and 24 months, all patients reported a clinically meaningful decrease in bowel QOL. Thus, only the timing of urinary and bowel toxicity appeared to differ among groups [14]. In a population-based study using Surveillance, Epidemiology, and End Results-Medicare-linked data comparing treatment of patients with localized prostate cancer using IMRT, 3D-CRT, or proton therapy, use of IMRT as compared to 3D-CRT was associated with less GI toxicity and fewer hip fractures but more erectile dysfunction. When IMRT was compared with proton therapy, use of IMRT was associated with less GI toxicity [15]. A meta-analysis including 20 published reports and 11,835 patients undergoing radiotherapy for prostate cancer showed when doses > 74 Gy were delivered, use of IMRT or proton beam radiotherapy was associated with a significant decrease in the rate of severe GI toxicity compared with 3D-CRT [16].
Treatment Techniques
Treatment Position and Immobilization Devices
For pelvic malignancies, in some cases, it may be advantageous to treat the patient in the prone position with studies showing a decrease in bowel dose and toxicity. This is especially relevant if IMRT is not being used such as in the case of definitive treatment of gynecological or GI malignancies.
Drzymala et al. compared the effect of the volume of bowel with dose received in the prone and supine positions in a prospective study of 19 patients with rectal cancer who underwent preoperative concurrent chemoradiotherapy using a three-field 3D-CRT technique to a total dose of 54 Gy in standard fractionation. For each patient, the planning target volume (PTV), bladder, and small bowel were outlined in both positions and the volume of bowel receiving doses in 5 Gy increments form 5–45 Gy was calculated using dose volume histogram (DVH). At 5 and 10 Gy dose levels, a significantly higher volume of bowel was irradiated in the supine position as compared to the prone position, at 15 Gy it was marginally significant and for 20–45 Gy there was no significant difference in the volume of bowel irradiated with each 5 Gy increment. Thus, radiotherapy delivered in the prone position for rectal cancer treatment may decrease small bowel dose for doses < 15 Gy, but may not provide an advantage for higher doses [17]. Koebl et al. showed similar results with a decrease in mean dose to the bladder and small bowel for patients undergoing postoperative radiotherapy for rectal cancer treated in the prone position as compared to the supine position [18].
Ghosh et al. compared volume of small bowel in the field in a prospective single-institution study in 21 cervical cancer patients who underwent adjuvant radiotherapy using a four-field 3D-CRT approach to a median dose of 47.8 Gy in the supine and prone positions with and without a belly board device. Patients were treated with a full bladder and abdominal radiographs with contrast were obtained to evaluate the volume of small bowel in the radiation fields. The belly board device was effective at minimizing the amount of small bowel in the lateral fields, whereas the prone position without the belly board device spared the smallest bowel in the anterior–posterior/posterior–anterior (AP/PA) fields. At median follow-up 37 months, there was no significant acute GI or GU toxicity and no patients experienced a small bowel obstruction [19].
Bladder Filling
Bladder filling has been shown to be an important factor to decrease GI and GU toxicity during elective pelvic nodal irradiation for prostate cancer. Jain et al. compared toxicity in a prospective single institution study of 230 patients undergoing whole pelvic radiotherapy using a four-field approach with a concomitant hypofractionated IMRT boost to the prostate as compared to IMRT applied to two different nodal volumes described as a 2 cm clinical tumor volume (CTV) margin around the pelvic vessels or by the RTOG prostate pelvic contouring atlas. Initially patients were treated with an empty bladder, with the remainder treated with a full bladder. The 4-field approach had significant higher rates of grade 3 urinary frequency (8 % vs 0 % vs 0 %) and significant grade 2 acute GI toxicity (31.9 % vs 20.8 % vs 7.2 %) as compared to the IMRT groups, respectively. Multivariate analysis suggested the factor that most influenced toxicity was bladder filling, followed by use of IMRT [20].
Treatment Planning
Organs at Risk Contouring
Radiation treatment planning is comprised of several steps; to begin, the radiation oncologist contours the tumor and target volumes and prescribes doses as well as contours critical structures that need to be avoided known as organs at risk (OAR). In the case of IMRT, the physicist then assigns dose constraints or restrictions on the dose to targets and OARs and importance factors for each. This information is then used by an optimization program to determine the treatment plan that best satisfies all the input criteria. By setting dose constraints for OARs, dose, and thus toxicity, can be decreased to adjacent normal tissue.
Bone marrow sparing to prevent hematological toxicity may be an important consideration in pelvic irradiation. Mahantshetty et al. conducted a phase II prospective study of 47 patients with cervical cancer treated with IMRT and concurrent chemotherapy. The authors compared volumes and DVH parameters for CT-based bone marrow contoured by two methods including whole bone and freehand inner cavity of bone and correlated these with ≥ grade 2 hematological toxicity. Various subvolumes were made for each set. Free-hand inner cavity volumes were 25–30 % of whole bone contours and were shown to be a better surrogate of active bone marrow on CT. There were significant differences between the DVH parameters of the two sets of subvolumes except for the V20 ischium, V10 sacrum, and V10 lumbosacral spine. Leukopenia, neutropenia, anemia, and thrombocytopenia > grade 2 was seen in 53, 29.8, 65.9, and 10.6 %, respectively. On both univariate and multivariate analysis, free hand inner cavity volumes V40 ≥ 40 % significantly correlated with ≥ grade 2 leukopenia and neutropenia with a statistically significant odds ratio (OR) of 4 [21].
Treatment Volume
The target volumes of importance consist of the gross tumor volume (GTV) comprising areas of gross tumor, CTV, or areas of microscopic disease and the PTV, which accounts for day-to-day motion and set-up inaccuracies. While the CTV is based on tumor type and risk of lymph node involvement along with other clinical or pathological risk features, larger treatment volumes, especially for pelvic malignancies, may lead to increases in toxicity.
De Jong et al. compared toxicity among 75 patients with endometrial carcinoma treated with adjuvant 3D-CRT using small (44 %), standard (37 %), or extended pelvic field (19 %). A small pelvic field including the central pelvis and proximal vagina was used for patients who had negative lymph nodes after adequate lymphadenectomy (> 10 lymph nodes), a standard field was used for patients with positive pelvic lymph nodes or inadequate lymphadenectomy and an extended pelvic field was used for patients with involved common iliac and/or para-aortic lymph nodes. Late GI toxicity was significantly different with 60.7 % versus 33.3 % in the standard and small field groups, respectively.
There were significant differences in acute side effects such nausea and anorexia with 32.1 and 3.0 % of patients in the standard versus small groups, respectively. There was also a significant difference in ileus with 14.3 and 0 % of patients in the standard versus small groups, respectively [22].
A secondary analysis of the RTOG 9413 randomized controlled trial of 324 prostate cancer patients who had a risk of lymph node involvement > 15 % who were randomized to whole pelvis or prostate-only or “mini” pelvis radiation therapy with neoadjuvant and concurrent hormonal therapy for a total of 6 months, correlated late toxicity with field size. Late grade 3 GI toxicity correlated with increasing field size in the “mini” pelvis versus prostate-only group while there was no correlation between field size and late grade 3 GU toxicity [23]. A single institution retrospective study correlated DVH parameters of the anal canal and rectum with rates of fecal incontinence for 44 prostate cancer patients who received 3D-CRT to 58–72 Gy versus 30 control patients. At a median of 1.5 years, there was worse incontinence in the radiation group with 27 and 14 % of patients reporting slight and severe incontinence, respectively, with continence similar among a range of doses administered. Severe incontinence was correlated with a significantly higher minimum dose to the anal canal, accompanied by portals extending significantly further inferiorly with respect to the ischial tuberosities [24].
Dose Constraints
Prevention of Hematological Toxicity
Radiotherapy of the pelvis involves treatment to a significant portion of the active adult bone marrow. Dose constraints to the pelvic bone marrow may be effective for decreasing hematological toxicity in patients undergoing concurrent pelvic chemoradiotherapy. Klopp et al. investigated hematological toxicity in 83 patients undergoing postoperative IMRT to 50.4 Gy with standard fractionation to the pelvic lymphatics and vaginal cuff with concurrent weekly cisplatin for cervical cancer patients in the prospective multi-institutional study RTOG 0418. The V10, V20, V30, and V40, as defined by a CT density-based auto-contouring algorithm, and median dose was correlated with hematological toxicity. 75 % of patients with a V40 > 37 % had ≥ grade 2 hematological toxicity, compared with 40 % of patients with a V40 ≤ 37 %, which was statistically significant. 74 % of patients with a median bone marrow dose > 34.2 Gy had ≥ grade 2 hematological toxicity compared with 43 % of patients with a dose of ≤ 34.2 Gy, which was also statistically significant. Thus, IMRT may make it possible to decrease the V40 and median dose to the bone marrow in order to decrease hematological toxicity [25].
Mell et al. showed in a retrospective single-institution review of 48 consecutive anal cancer patients who underwent concurrent chemoradiotherapy using IMRT to a median dose of 45–50.4 Gy in standard fractionation, 56, 50, 8, and 27 % of patients experienced grade 3–4 leukopenia, neutropenia, anemia, and thrombocytopenia, respectively. On multiple regression analysis, V5, V10, V15, and V20 were significantly associated with decreased white blood cell (WBC) and absolute neutrophil count nadirs, as well as female gender, decreased BMI, and increased lumbosacral bone marrow receiving 10, 15, and 20 Gy. Lymph node positivity was also significantly associated with a decreased WBC nadir on multiple regression analysis. Thus, increased low-dose radiation to pelvic bone marrow was associated with acute hematologic toxicity during chemoradiotherapy for anal cancer and techniques to limit bone marrow irradiation may reduce hematologic toxicity [26].
Prevention of Genitourinary Toxicity
Studies have shown, the bladder V78–80 is the most predictive of late GU toxicity in patients undergoing radiotherapy for prostate cancer. Dose volume modeling of bladder toxicity is sparse, and may indicate the difficulty in assessing bladder toxicity given wide variations in bladder dose due to variable filling [27].
Prevention of Gastrointestinal Toxicity
Pederson et al. in a retrospective single-institution study of 296 patients treated with IMRT for prostate adenocarcinoma to a median dose of 76 Gy to the prostate with or without proximal seminal vesicles, correlated bladder, and rectum V70, V65, and V40 with maximal ≥ grade 2 GU and GI toxicity. At a median follow-up of 41 months, there was 9 % and 5 % ≥ grade 2 GU and GI toxicity, respectively. Freedom from ≥ grade 2 GU and GI toxicity at 4 years was 100 % for patients with rectal V70 ≤ 10 %, V65 ≤ 20 %, and V40 ≤ 40 %; 92 % for patients with rectal V70 ≤ 20 %, V65 ≤ 40 %, and V40 ≤ 80 %; and 85 % for patients exceeding these criteria. Age ≥ 70 years had a higher associated with GI toxicity. There were no bladder dose–volume relationships observed for risk of GU toxicity [28].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree