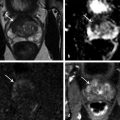
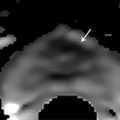
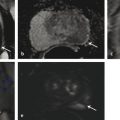
American Joint Committee on Cancer (AJCC) clinical TNM staging classification of prostate cancer. Stage definition Primary tumor (T) clinical Tx Primary tumor cannot be assessed T0 No evidence of primary tumor T1 Clinically inapparent tumor neither palpable nor visible by imaging T1a Tumor incidental histologic finding in 5% or less of tissue resected T1b Tumor incidental histologic finding in more than 5% of tissue resected T1c Tumor identified by needle biopsy (for example, because of elevated PSA) T2 Tumor confined within prostatea T2a Tumor involves one-half of one lobe or less T2b Tumor involves more than one-half of one lobe but not both lobes T2c Tumor involves both lobes T3 Tumor extends through the prostate capsuleb T3a Extraprostatic extension (unilateral or bilateral) T3b Tumor invades seminal vesicle(s) T4 Tumor is fixed or invades adjacent structures other than seminal vesicles, such as external sphincter, rectum, bladder, levator muscles, and/or pelvic wall Regional lymph nodes (N) clinical NX Regional lymph nodes were not assessed N0 No regional lymph node metastasis N1 Metastasis in regional lymph node(s) Distant metastasis (M)c clinical M0 No distant metastasis M1 Distant metastasis M1a Nonregional lymph node(s) M1b Bone(s) M1c Other site(s) with or without bone disease Abbreviation: PSA, prostate-specific antigen. Source: Adapted from the AJCC Cancer Staging Manual, 7th edition, 2010.3 a Tumor found in one or both lobes by needle biopsy but not palpable or reliably visible by imaging is classified as T1c. b Invasion into the prostatic apex or into (but not beyond) the prostatic capsule is classified not as T3 but as T2. c When more than one site of metastasis is present, the most advanced category is used. pM1c is most advanced, where “p” stands for “prostate”. Pelvic lymph node metastases have a significant impact on the prognosis of patients with malignancies. For example, even micrometastases in a single node are generally considered to rule out surgical cure by the available treatment protocols in prostate cancer patients. 4 The status of the lymph nodes largely dictates the management of the primary tumor. In patients with low-risk prostate cancer, the rate of lymph node involvement is low, ranging between 0.5 and 0.7%. 5, 6 In patients with stage T2 disease, lymph node dissection reveals lymph node metastasis in 10 to 25% of cases. Recent data suggest that prostate cancer patients with minimal lymph node involvement can be cured by extended pelvic lymph node dissection when radical prostatectomy is performed as initial therapy. 7 Imaging has become an indispensable tool in cancer research, clinical trials, and medical practice. Magnetic resonance (MR) imaging (MRI) is the most widely used cross-sectional imaging technique for prostate cancer. While ultrasound provides real-time data, it is also highly operator dependent and experience is needed in order to perform it well. Magnetic resonance (MR) imaging allows for a more standardized examination of the prostate and with the addition of functional imaging techniques such as diffusion-weighted MR imaging, proton MR spectroscopic imaging, and dynamic contrast-enhanced MR imaging, a unique insight can be obtained into the cancer characteristics. This chapter focuses on the role of MR imaging in the assessment of extraprostatic extension, seminal vesicle invasion, and lymph node metastases, as well as in surgical planning for nerve-sparing procedures. Clinical staging of prostate cancer currently entails the use of digital rectal examination, prostate-specific antigen (PSA) measurement, as well as transrectal ultrasound. The clinical stage is identified using these variables and expressed using the TNM staging classification ( ▶ Table 7.1). Stage T1a and T1b tumors cannot be identified by digital rectal examination of the prostate. They are found incidentally in prostatic tissue removed during transurethral resection or during prostatectomy performed for benign prostatic hypertrophy. Tumors of these stages are generally referred to as “incidental carcinomas.” These tumors are found in 8 to 12% of patients undergoing surgery for benign disease. 8, 9 Patients rarely die from T1a or T1b disease but rather from other age-related causes. Prostate cancer diagnosed by needle biopsy after an elevated PSA is termed stage T1c disease if both the digital rectal examination is normal and no lesions are visible on the transrectal ultrasound. In disease stages T2a through T2c, there is either an organ-confined palpable nodule on digital rectal examination or evidence of one or multiple tumors on imaging. This category of prostate cancers is potentially curable. In patients with stage T2 disease, lymph node dissection reveals lymph node metastasis in 10 to 25% of cases. 10 The natural history of T2 prostate cancer has been shown to be associated with a 10-year rate of local progression in 66% of patients diagnosed and progression to metastatic disease in 33% of patients diagnosed. 11 Stage T3 prostate cancers have extraprostatic extension and a much poorer prognosis compared to organ-confined disease. However, since radical prostatectomy offers promising oncologic outcomes in patients with pathologic T3 disease, preoperative MRI could be of help in both predicting the presence of extraprostatic extension and in providing information about the location of extraprostatic extension for surgical planning. Depending on the depth of extraprostatic involvement, lymph node metastases occur in up to 50% of these cases. Because digital rectal examination and PSA have been shown to be of limited value in the prediction of stage T3 tumors, numerous imaging modalities have been applied to improve local staging accuracy. Computed tomography (CT), positron emission tomography (PET), as well as MR imaging, have been used to improve the prediction of stage T3 disease. In the event of lymph node involvement, prognosis is determined by the N status rather than by the T category. It has been shown that cure rates with surgery alone are not to be expected to exceed 30%. 12 During pelvic lymphadenectomy, metastatic lymph nodes are identified to various degrees. Median time to progression in this group is on the order of 11 to 24 months. In terms of survival, it seems to be of little importance whether hormonal treatment is deferred or started immediately. The reported median time to progression can be prolonged up to 5 years with early hormonal treatment, although this is achieved at the cost of side effects. Local staging is accomplished by examining the prostatic capsule and seminal vesicles. Multiparametric prostate MR imaging is currently the most accurate imaging modality to preoperatively stage prostate cancer. 13 Magnetic resonance imaging has a higher accuracy in the assessment of intraprostatic disease (stage T2; ▶ Fig. 7.1), extraprostatic extension ( ▶ Fig. 7.2), and seminal vesicle invasion (stage T3; ▶ Fig. 7.3) as well as invasion of peri-prostatic structures (stage T4; ▶ Fig. 7.4), compared to other imaging techniques. In patients in whom the diagnosis of cancer has been established, reliably determining its local stage, along with localization of tumor within the gland, is an important element of prostate MR imaging. 14 In the decade prior to this writing, the focus of MR imaging in prostate cancer moved from staging to localization of the disease. Information regarding location of the tumor, capsule involvement, tumor volume, and neurovascular bundle integrity are becoming more important than “simple” stage information. With improvements in surgical techniques, it is now possible to combine all of this information in the surgical planning. Fig. 7.1 A 63-year-old man with biopsy-proven bilateral prostate cancer (Gleason score 3 + 4 =7) in peripheral zone of the midprostate and a prostate-specific antigen level of 6.8 ng/mL. The axial T2-weighted endorectal-coil MRI shows bilateral well-circumscribed low–signal-intensity areas (white arrows) confined to the prostate (stage T2 prostate cancer). Fig. 7.2 A 74-year-old man with biopsy-proven prostate cancer (Gleason score 3+4 tumor in 6 out of 6 biopsy cores from the right lobe; benign in 6 out of 6 cores from the left lobe.) and a prostate-specific antigen level of 28 ng/mL. Digital rectal examination showed stage T2 prostate cancer. The patient underwent staging T2-weighted MRI without an endorectal coil, which revealed a large mass in the right half of the prostate with clear evidence of extraprostatic extension (white arrows) (stage T3a prostate cancer). Fig. 7.3 A 3-T T2-weighted MRI of the prostate in a 66-year-old man with a prostate-specific antigen level of of 9.5 ng/mL and biopsy-proven prostate cancer with a Gleason score of 4+3 in the left prostate. A low–signal-intensity lesion (white arrows) is visualized in the left seminal vesicle consistent with seminal vesicle invasion (stage T3b prostate cancer). Fig. 7.4 A 71-year-old man with a prostate specific antigen level of 9.3 ng/mL and Gleason score 4 + 4 = 8 prostate cancer underwent staging MRI. The T2-weighted axial image shows a large bulky tumor (T) in the right prostate gland with evidence of invasion into the right puborectal sling (dashed arrows) and possible invasion into the rectal wall (white arrows) (stage 4 prostate cancer; invasion of periprostatic structures). To justify the use of an expensive imaging modality such as MR imaging, patients’ outcome should be improved by preoperative staging. 13 To achieve this goal, staging accuracy should be high, the results should affect diagnostic and especially therapeutic thinking, and the alternative therapy should increase life expectancy and quality of life. In local staging, T2-weighted MR imaging ( ▶ Fig. 7.5) is the most important sequence. T2-weighted MR imaging has the highest in-plane spatial resolution compared to the other imaging sequences included in prostate MR imaging (i.e., DWI, MRSI, and DCE-MRI), and is therefore crucial in capsular and neurovascular bundle involvement assessment. However, it is not possible to state a single overall accuracy of MR imaging for staging prostate cancer because of a very wide divergence across published studies. 15 In a meta-analysis, the reported summary receiver operating characteristic curve for MR imaging in prostate cancer local staging (T2 vs. T3) had a joint maximum sensitivity and specificity of 71 to 74%. 15, 16 However, this summary estimate is limited given the heterogeneity in staging performance across centers, such that the staging performance of MR imaging in one’s local practice is likely to differ. Furthermore, due to incomplete reporting within individual studies, it is not possible to fully explain the basis of this heterogeneity of staging performance in the literature. Nonetheless, it was suggested that use of turbo spin-echo imaging using an endorectal coil and multiple imaging planes improved staging performance. 15 Fig. 7.5 An axial T2-weighted MRI of the prostate in a healthy man. The peripheral zone (PZ) has a higher signal intensity than the transition zone (TZ). The rectum (R) is located posterior to the prostate. The capsule is annotated with white arrows. Magnetic resonance (MR) imaging sequences performed at low field strengths with the conventional body coil or phased-array surface coils lack sufficient resolution and signal-to-noise ratio to identify fine anatomical details of the prostate gland and periprostatic tissues necessary for accurate staging. 17 However, improvements in coil technologies, higher field strength, and sequence development have led to higher staging accuracies. Currently, endorectal 3-T MRI can be considered the most reliable noninvasive technique for the local staging of prostate cancer. 13, 18, 19, 20, 21 The most cost-effective group of patients to undergo local staging with endorectal MR imaging are those considered to have an intermediate risk of T3 prostate cancer, based on a PSA level between 4 and 20 ng/mL and a Gleason score between 5 and 7. 22 In this group of patients, endorectal MR imaging is useful because the decision on selection of treatment (prostatectomy or a form of radiation therapy and/or hormonal deprivation) is most likely to depend on the imaging results. Inclusion of MR imaging results in clinical nomograms that help improve the prediction of cancer extent, thereby improving patient selection for local therapy. 23 Ideally, prostate MR imaging should have a low percentage of false-positives for extraprostatic extension to ensure that few, if any, patients will be deprived of potentially curative treatment options. Historically, it has been suggested that all patients who are considered candidates for a radical treatment should undergo MR imaging with a high specificity interpretation in order to guide the final treatment selection. 19 However, this conventional thinking is changing due to a combination of factors. First, nerve-sparing radical prostatectomy is now commonly applied as the routine surgical approach to localized prostate cancer. In addition, urologists now more commonly will consider performing radical prostatectomy in patients with suspected extraprostatic extension of prostate carcinoma (EPE), albeit with a wider surgical margin than in other patients. Thus, higher sensitivity for EPE on MRI in such contexts may be appropriate, given that an overcall of EPE would not preclude surgery in such centers and in order to reduce the risk of positive margins in those patients with EPE who do proceed to surgery. Prostate MR imaging should be obtained at least 4 to 6 weeks after image-guided biopsy, given that postbiopsy hemorrhage ( ▶ Fig. 7.6) decreases not only the localization accuracy but also the staging accuracy as well. 24, 25, 26 Nonetheless when extensive postbiopsy change is present, the distribution of hemorrhage can be used to assist tumor detection. Namely, areas that are excluded from the otherwise extensive hemorrhage, when also showing a corresponding area of homogeneous low signal intensity at T2-weighted MR imaging, are likely to represent cancer. 27 Fig. 7.6 Areas of high signal intensity (white Hs) representing biopsy hematoma on a T1-weighted MRI in the right and left peripheral zone. In general, a combination of a pelvic phased-array coil and endorectal coil are used at a field strength of 1.5T, while either the pelvic phased-array, endorectal coil, or a combination are used at 3T. At both 1.5T and 3T, endorectal coils ( ▶ Fig. 7.7; ▶ Fig. 7.8) have been shown to improve prostate MR image quality and staging performance compared with those of pelvic phased-array coils, although the necessity of an endorectal coil at 3T is debatable. 28 Patients tolerate the endorectal coil well, although the insertion remains uncomfortable. 29 For the endorectal coil, the primary potential adverse effect on imaging is an increase in the incidence of bowel motion artifacts, which deteriorate image quality. 21 In the European Society of Urogenital Radiology prostate MRI guidelines, the combination of endorectal coil and pelvic phased-array coil are recognized to provide excellent signal-to-noise ratio and to be considered state-of-the-art imaging. 30 Fig. 7.7 An axial T2-weighted endorectal-coil MRI. Note the signal drop at the anterior part of the prostate and pubic bone (arrow). Fig. 7.8 A sagittal T2-weighted endorectal-coil (ERC) MRI. Artifacts are present related to the coil and bowel motion (arrows). However, these artifacts do not decrease the image quality of the prostate itself due to the head-to-feet readout of MRI acquisition. The imaging protocol consists of high in-plane resolution T2-weighted sequences in at least 2 planes, as well as DWI and DCE-MR imaging in the axial plane with preferably the same slice thickness and slice gap as the T2-weighted anatomical imaging sequence. T2-weighted imaging provides the best depiction of the prostate’s zonal anatomy and capsule 31 ( ▶ Fig. 7.5; ▶ Fig. 7.9). Anatomical T2-weighted MR imaging is obtained with a small field of view covering the entire prostate and seminal vesicles. There is no evidence to support the usefulness of fat suppression for T2-weighted sequences. Indeed, use of frequency-selective fat suppression does not significantly improve the diagnosis of extraprostatic disease and decreases the signal-to-noise ratio, which may limit visualization of anatomical details and reduce the definition of the prostatic capsule. Moreover, suppression of fat signal intensity leads to the reduced definition of periprostatic anatomical planes and degrades the visualization of structures within the prostatic fat, such as the neurovascular bundles. Contrast between extraprostatic tumor and periprostatic fat may also be reduced. 32 Fig. 7.9 An axial T2-weighted MRI of a 75-year-old man with histopathologically proven prostate cancer (Gleason score 4 + 3 = 7) in the right peripheral zone (arrows). The prostate capsule is a thin layer on T2-weighted imaging (arrowheads). Among locally invasive tumors, the distinction between those penetrating the prostate capsule but sparing the seminal vesicles (stage T3a) and those invading the seminal vesicles (stage T3b) is important in patient prognosis and therapeutic planning. 33 In addition, according to the TNM staging system, tumors invading but not penetrating the capsule are classified as T2 and not as T3 disease. 34 On T2-weighted images, extraprostatic extension can be detected by visualizing the direct extension of the tumor into the periprostatic fat. Indirect imaging criteria for the detection of EPE include asymmetry of the neurovascular bundle ( ▶ Fig. 7.10; ▶ Fig. 7.11), obliteration of the rectoprostatic angle, tumor bulge into the periprostatic fat ( ▶ Fig. 7.12), broad tumor contact with the surface of the capsule (> 1.5 cm) ( ▶ Fig. 7.13), and capsular retraction 35, 36, 37, 38 ( ▶ Table 7.2). Despite the development of these indirect criteria, the sensitivity and specificity for local staging with MRI vary considerably with technique and population: 14.4 to 100% and 67 to 100%, respectively. 36 This heterogeneity may also reflect that the accuracy for local staging can be influenced by the extent of EPE that is present, with MR imaging having high accuracy for established EPE, although more limited accuracy for focal or localized EPE. False-negative interpretations for EPE may occur in the presence of microscopic EPE, and false-positive interpretations for EPE may occur due to normal variation and heterogeneity in the appearance and degree of visualization of the capsule between patients ( ▶ Fig. 7.14; ▶ Fig. 7.15). Given these considerations, the definitive diagnosis of EPE on MRI should only be made when direct and/or gross EPE is visualized. In comparison, the diagnosis of EPE should only be suggested when just the previously noted secondary and/or indirect findings are present. Fig. 7.10 A 64-year-old man with stage T3a prostate cancer in the left peripheral zone (PZ) though with minimal extraprostatic extension. T2-weighted MRI shows that the tumor (T) has lower signal intensity than the PZ. It also shows the asymmetry of the left rectoprostatic angle (arrowhead) and some capsular bulging (arrows). Fig. 7.11 A 71-year-old man with stage T3a disease in the left peripheral zone (PZ) (Gleason score 5 + 3 = 8). The T2-weighted MRI shows that the tumor (T) has lower signal intensity than the PZ and that there is bulging of the capsule (arrows).
7.2 Staging of Prostate Cancer
7.3 Local Staging





7.3.1 Acquisition Protocol



7.3.2 T2-Weighted MR Imaging



Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree