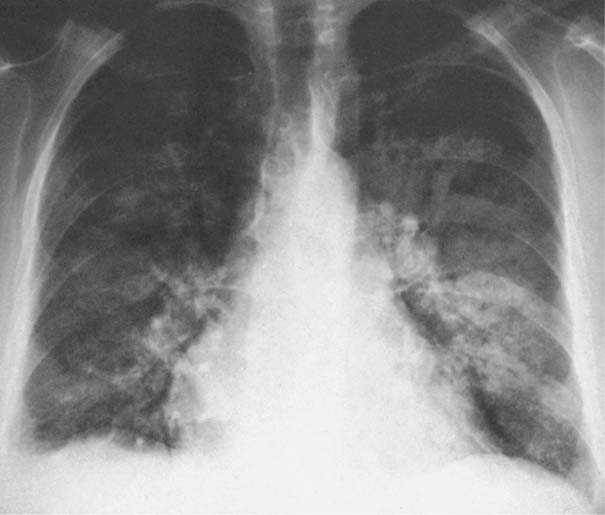
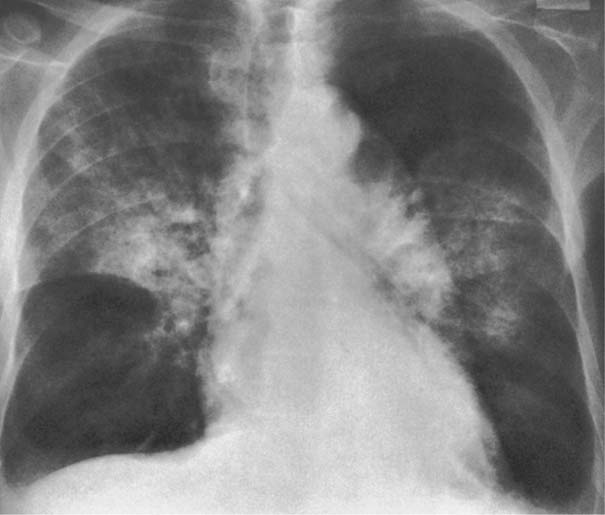
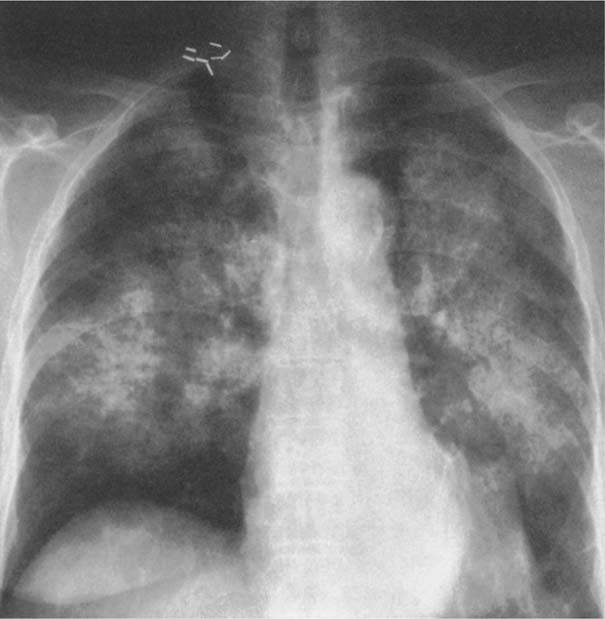
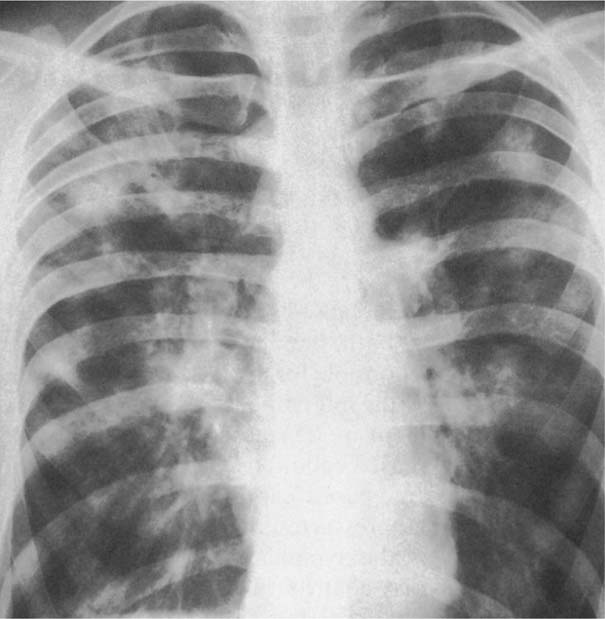
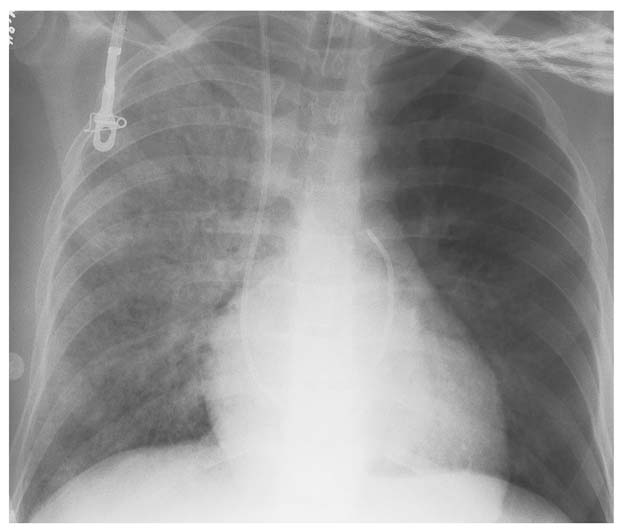
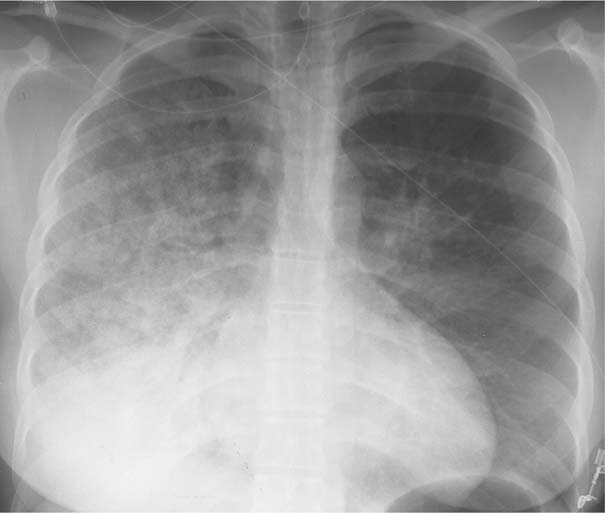
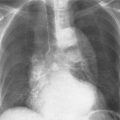
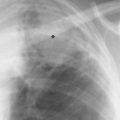
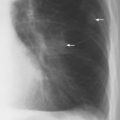
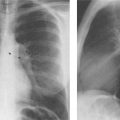
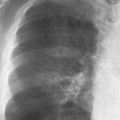
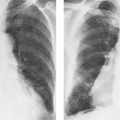
7 Pulmonary Edema and Symmetrical Bilateral Infiltrates Pulmonary edema is caused by the accumulation of excessive fluid in both the interstitial and alveolar spaces. The two main factors responsible for the leak of fluid from the capillary space into the interstitial and subsequently the alveolar compartments are an elevated capillary blood pressure and increased capillary permeability. A decrease in either serum osmotic pressure or interstitial fluid pressure can contribute to the development of pulmonary edema, although these abnormalities are unlikely to cause edema by themselves. Other factors contributing to the development of pulmonary edema include a decrease in alveolar pressure and surface tension as well as obstruction of the lymphatics draining the lung interstitium, but their significance in this pathophysiologic mechanism is not clearly determined yet. The most common cause of pulmonary edema resulting from an increased capillary pressure (hydrostatic pulmonary edema) is left ventricular failure (cardiogenic edema). Pulmonary veno-occlusive disease (e.g. idiopathic, congenital pulmonary vein anomalies, invasion or compression of pulmonary veins) may also induce hydrostatic pulmonary edema. An elevated pulmonary capillary pressure associated with a decreased serum osmotic pressure is responsible for pulmonary edema associated with renal failure and fluid overload. Neurogenic pulmonary edema results from a combination of both elevated capillary blood pressure and increased capillary permeability. Pulmonary edema caused by increased capillary permeability is frequently referred to as adult respiratory distress syndrome (ARDS). It is associated with sepsis, overwhelming pneumonia, aspiration, inhalation of noxious gases, pulmonary contusion, fractures (e.g. long bones and pelvis), near-drowning, burns, blood transfusions, major surgery (e.g. coronary bypass), prolonged hypotension, disseminated intravascular coagulation, and drug overdose. Pulmonary edema is radiographically characterized by a bilateral, diffuse increase in interstitial markings with a loss of definition and by fluffy confluent opacities representing the alveolar process. This radiographic pattern is found most commonly in cardiogenic pulmonary edema, where the increased capillary blood pressure causes an abnormal plasma leak into the interstitial and alveolar spaces (Fig. 7.1). The edema accumulates predominantly in the most dependent portions of the lungs. In the upright position, this is in the mid- and, particularly, lower lung fields, whereas in the bedridden patient with the radiograph taken in the supine position, the edema appears more evenly distributed throughout both lungs. An unusual distribution pattern of the pulmonary edema might, however, be found with a pre-existing chronic lung disease (e.g. emphysema), where the edema spares the most severely damaged parts of the lungs (Fig. 7.2). Bronchogenic spread of various exogenous and endogenous materials and organisms may also cause diffuse alveolar infiltrates that may or may not be associated with interstitial disease. Finally, the infiltration of the alveolar and interstitial space with inflammatory or neoplastic cells can produce a radiographic appearance that also simulates pulmonary edema. An unusual presentation of pulmonary edema takes the form of the “bat’s wing” or “butterfly pattern,” in which the hilar and perihilar areas of the lungs are fairly dense and uniformly consolidated and the peripheral 2–3 cm of the lung parenchyma are relatively uninvolved (Fig. 7.3). This pattern is relatively frequently seen with uremia, alveolar proteinosis, and pneumocystis carinii pneumonia, and with conditions causing pulmonary hemorrhage, such as idiopathic pulmonary hemosiderosis and polyarteritis nodosa. However, it is not at all pathognomonic of these conditions and can be found with virtually every disease known to produce a pulmonary edema pattern. Fig. 7.1 Cardiogenic pulmonary edema. Cardiomegaly, bilateral interstitial and alveolar infiltrates involving predominantly the mid- and lower lung fields, and small pleural effusions are seen. This acute edema was caused by a left atrial myxoma that has suddenly enlarged secondary to intratumoral bleeding. Fig. 7.2 Cardiogenic pulmonary edema in chronic obstructive pulmonary disease. Asymmetric distribution of the pulmonary edema that spares the parts of the lungs with the most severe emphysematous changes is seen. Fig. 7.3 Pneumocystis carinii pneumonia in compromised host. The pulmonary infiltrates, consisting of an interstitial (reticulonodular) and alveolar component, assume a “bat’s wing” or “butterfly pattern,” sparing the peripheral 2–3 cm of the lung parenchyma. Fig. 7.4 Loeffler’s syndrome (acute eosinophilic pneumonia). Bilateral patchy consolidations in the lung periphery parallel to the lateral chest wall are characteristic (“reversed pulmonary edema pattern”). The more central appearing infiltrates are anatomically located in the anterior or posterior lung periphery. Fig. 7.5 Aspiration. A unilateral pulmonary edema pattern with air bronchograms is seen in the right lung. The aspiration occurred with the patient lying on his right side. Fig. 7.6 Narcotic abuse (cocaine). Pulmonary edema is present bilaterally, but much more severe on the right side. The “reversed pulmonary edema pattern” represents virtually a photographic negative of the “bat’s wing” or “butterfly” pattern and is characterized by homogeneous consolidations in the lung periphery running more or less parallel to the lateral chest wall. This pattern is commonly found in acute (Loeffler’s syndrome) and chronic eosinophilic pneumonia (Fig. 7.4). An edema pattern caused by pulmonary hemorrhage frequently appears somewhat more dense than usual, although this finding largely depends on the employed radiographic technique. It may be observed with lung contusion, bleeding or clotting disorders, idiopathic pulmonary hemosiderosis, Goodpasture syndrome, systemic lupus erythematosus and chronic renal failure. Unilateral pulmonary edema (Fig. 7.5 and 22.6) can be divided into ipsilateral and contralateral types. The former refers to conditions in which the pathogenetic mechanism leading to the asymmetry is on the side of the edema and include prolonged lateral decubitus position in cardiac decompensation, unilateral aspiration, pulmonary contusion, rapid thoracentesis, and unilateral bronchial or pulmonary venous obstruction. Contralateral pulmonary edema refers to accumulation of excess water in the normal lung opposite the diseased lung. The most common cause is chronic obstructive pulmonary disease (COPD), but it is also associated with acute pulmonary thromboembolism, Swyer–James syndrome, and unilateral lung destruction, fibrosis and pleural disease.
Disease | Radiographic Findings | Comments |
Bronchioloalveolar carcinoma (alveolar cell carcinoma) (Fig. 7.7) | Alveolar infiltrates combined with reticulonodular and linear densities. | Pleural effusions in approximately 10%. Hilar and mediastinal lymph node enlargement uncommon. |
Lymphangitic carcinomatosis (Fig. 7.8) | Interstitial and alveolar infiltrates similar to cardiogenic pulmonary edema, but with severe loss of lung volume and without cardiomegaly. Pleural effusions are commonly associated. | This represents an advanced stage of the disease that is virtually always associated with severe dyspnea. |
Lymphoma and leukemia (Fig. 7.9) | Bilateral interstitial and alveolar infiltrates involving preferentially the perihilar areas and lower-lung fields. Appearance of symmetrical lung involvement may vary from predominantly interstitial edema to homogeneous consolidations. | In a lymphoma or leukemia patient these findings are, however, more often caused by intervening pneumonias, drug reaction, or hemorrhages, rather than by the underlying malignancy itself. |
Kaposi’s sarcoma (Fig. 7.10) | Numerous poorly defined metastases may mimic extensive bilateral infiltrates (opportunistic infections). | Common in male homosexual AIDS patients. |
Pneumonia, bacterial (e.g., staphylococcus, Gram-negative bacteria, anaerobics, and tuberculosis) (Fig. 7.11) | Patchy confluent infiltrates often associated with areas of homogeneous consolidations. Cavitary lesions are relatively common and their demonstration is useful for the differentiation from other conditions presenting with a pulmonary edema pattern. | Bronchogenic spread by inhalation (e.g., staphylococcus), aspiration (e.g., anaerobic bacteria) or communication between abscess or cavity and bronchial system (e.g., tuberculosis). |
Pneunomia, fungal (e.g., histoplasmosis, coccidioidomycosis, blastomycosis, aspergillosis, candidiasis) | Bilateral confluent infiltrates similar to aforementioned bacterial pneumonias, but cavitation less common. Hilar lymph node enlargement occurs only rarely, but when present, might be useful for differential diagnosis from nonfungal pneumonias. | This form is virtually limited to compromised hosts. An overwhelming exposure to the fungus rarely may produce this radiographic appearance in histoplasmosis. |
Pneumonia, mycoplasma and viral (influenza, parainfluenza, coxsackie, adenovirus, psittacosis, varicella, SARS) (Fig. 7.12) | Diffuse reticular pattern with superimposed patchy alveolar infiltrates. Cavitation does not occur and hilar lymph node enlargement is extremely rare in the adult. | SARS (severe acute respilatory distress syndrome) presenting with a pulmonary edema pattern (15%) is associated with the highest mortality rate. Rickettsial infections (e.g. Q-fever and Rocky Mountain spotted fever) may occasionally mimic viral pneumonias. |
Cytomegalovirus (cytomegalic inclusion disease, CID) (Fig. 7.13) | Diffuse reticulonodular and alveolar infiltrates, preferentially involving the periphery of the middle and lower lobes. | In compromised hosts (e.g., renal transplants). |