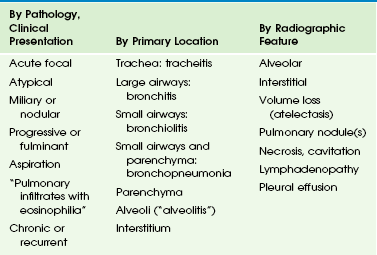
Chapter 54 Pneumonia and other pulmonary infections, defined as those involving the lower respiratory tract below the glottis, continue to be the most common cause of illness in children, affecting over 150 million children under the age of 5 years per year worldwide, and are implicated in 20 million hospitalizations annually in the United States.1–5 Since clinical signs and symptoms are poor predictors of pediatric pulmonary infections, and the value of microbial studies is limited, chest radiography with the use of standardized reporting criteria continues to be the best available diagnostic standard.1,3,6,7 The value of lateral radiograph in diagnosis continues to be debated, but the hyperinflation that is a radiographic hallmark of pulmonary infections in young children is more reliably detected on the lateral radiograph than on the frontal radiograph.1,8,9 In the ambulatory care setting, performing routine chest radiography has not been shown to improve the outcomes of pulmonary infections in young children, and it is not indicated in first-time wheezing episodes presumed to be viral or reactive in etiology.10–12 The yield of radiography is greater in the presence of a high temperature and in the absence of a family history of asthma.13 Radiography is most helpful when an inconsistency exists among the data from history, physical examination, and observation.14 Negative chest radiography provides justification to withhold antibiotics in symptomatic children.1 Valid indications for chest radiography, therefore, are severe disease, confirmation or exclusion of diagnosis in the presence of an atypical presentation, assessment of complications, and exclusion of other causes of respiratory distress.10 Maternal antibodies protect newborns against viral pulmonary infections, and bacterial infections are most common in this age group, usually caused by pathogens acquired during labor and delivery. With dropping maternal antibodies, viral infections become more prevalent between ages 2 months and 2 years. After this, bacterial infections again become more common.3 Tuberculosis, fungal infections, and parasitic infestations continue to add substantially to the disease burden of children who are immunocompromised or live in endemic areas.2 The spectrum of pulmonary infections in childhood is categorized in Table 54-1.2,15 However, the distinctions between these categories are arbitrary, since considerable overlap exists both at presentation and during evolution of disease. In young children, the lungs can only respond to insult in a limited number of ways, and this response is more age specific than antigen dependent.16–20 Viral and bacterial infections frequently coexist, and radiographic criteria alone do not reliably distinguish between them.21–23 This is compounded by a reported high interobserver variability for interpretation of chest radiographs.24–27 Use of inexact terminology may hamper communication between radiologists and referring physicians, who agree with radiologists’ interpretations in only 78% of cases, and antibiotics are frequently prescribed even when no bacterial agent can be proven.6,28,29 Table 54-1 Classification of Pulmonary Infections in Childhood From Eslamy HK, Newman B. Pneumonia in normal and immunocompromised children: an overview and update. Radiol Clin North Am. 2011;49:895-920. Viral pulmonary infections usually occur after the inhalation of infected air droplets.2 The clinical presentation depends on the infectious agent, patient age, and immune response (mainly cellular, T-cell–mediated immunity). In young children, degrees of mucosal swelling within the small-caliber terminal airways, which would not compromise air exchange in older individuals with relatively larger-caliber airways, lead to diffuse alveolar air trapping. This, in combination with lack of development of collateral pathways of ventilation via the pores of Kohn and canals of Lambert, leads to fixed hyperinflation of the lungs (Fig. 54-1). In addition, more hypersecretion occurs in the inflamed airways in young children, contributing to mucous plugging of the airways; this leads to (sub)segmental atelectasis mimicking alveolar consolidations, which are frequently misinterpreted to represent bacterial pneumonia.1,29–31 Figure 54-1 Respiratory syncytial virus pneumonia in a 1.5-year-old boy. Etiology: The most common viral agents causing pulmonary infections in childhood are listed in Table 54-2. RNA Viruses: Respiratory syncytial virus (RSV) consists of 10 genes encoding 11 proteins that are associated with inhibiting type 1 interferon activity. It is the most common cause of pulmonary infections in infants and young children. The disease can be virulent and is fatal in up to 1% of healthy infants, but those with chronic lung disease from prematurity and cardiovascular disease are at much higher risk.32 Clinical signs range from mild coryza to severe respiratory distress with wheezing, tachypnea, cyanosis, dyspnea, and retractions. Hypoxemia, possibly caused by a ventilation–perfusion imbalance, may be profound and last for several weeks.33 The diagnosis of RSV infection is made by examining the nasoepithelial cells by using direct fluorescent antibody detection. Human metapneumovirus (HMV) is a negative single-stranded ribonucleic acid (RNA) virus of the family Paramyxoviridae, and the second most common cause of viral pulmonary infections in young children after RSV.34 It affects children who are slightly older than those infected by RSV, and the disease is less severe, except when seen as a coinfection. HMV has a worldwide distribution and causes a disease spectrum indistinguishable from influenza and RSV infection, with the same seasonal variation. The various subtypes of the influenza virus are common causes of pneumonia requiring hospitalization in young and school-age children, ranking behind RSV and parainfluenza. Influenza attacks the ciliated respiratory epithelium, and lesions may extend to the distal airways, producing severe pneumonia. The onset is often more abrupt and intense than that of RSV or parainfluenza. The newly isolated avian influenza virus (H1N1), originating in Asia from infected poultry, has spread over many parts of the world from 2003 to 2007.34–36 A novel H1N1 infection resulting from antigenic shift of the virus has been reported since 2009 and has been associated with a higher rate of shock, acute respiratory distress syndrome (ARDS), and neurologic complications in children compared with seasonal (non-H1N1) infections.37 Severe acute respiratory distress syndrome (SARS) is caused by a coronavirus, which was recognized in 2003, and in the following year, it was diagnosed in over 8000 patients, primarily in China, Taiwan, Hong Kong, Vietnam, and Toronto.34,38 After 2004, the outbreak appeared to have been contained; since then, SARS has only been sporadically reported. Like most respiratory viral diseases, it is spread by face-to-face contact. SARS typically has a brief prodrome of fever, with or without constitutional symptoms. This quickly progresses to severe respiratory symptoms by day 6 of the fever.39 Most patients require hospitalization. It has an overall mortality of 10%, but children have constituted only 5% of reported cases, and no pediatric deaths have been reported.34 Children have a mild clinical course and recover with no sequelae.38 Recently, newer coronaviruses (NL63 and HKU1), which cause milder forms of respiratory disease, have been discovered.34 DNA Viruses: Adenovirus pneumonia is responsible for about 5% of respiratory tract disease in infants and children, with a peak age between 6 months and 5 years.31 It is a common cause of viral pneumonia, along with RSV, parainfluenza virus, and influenza virus. Adenovirus has also been associated with a pertussis-like syndrome. Although often relatively benign, adenoviral infection may be severe and even fatal in young infants, particularly when caused by the recently identified serotype Ad14.34 Imaging: Bilateral interstitial opacities with peribronchial thickening and hyperinflation (see Fig. 54-1), thought to represent viral bronchiolitis, are nonspecific and are, in fact, indicative of an acute pulmonary infection of any cause (viral or bacterial) in young children.1,15 Pleural effusions are rare in purely viral lung infections. Radiologic abnormalities often clear slowly and lag behind clinical improvement. Complications are superimposed bacterial infection (often hospital-acquired), and postinfectious bronchiolitis obliterans, bronchiectasis, or both.2 These latter conditions, which frequently follow an adenovirus infection, are characterized by features of chronic air trapping and atelectasis resulting from bronchial dysfunction; mosaic perfusion abnormalities, peribronchial thickening, chronic atelectasis and bronchiectasis, best seen on computed tomography (CT).2 Swyer-James-MacLeod syndrome is characterized by a unilateral small hyperlucent lung, which exhibits hypoperfusion and chronic bronchiectasis (e-Fig. 54-2). e-Figure 54-2 Swyer-James-MacLeod syndrome in a 20-year-old young man monitored since early childhood; he had chronic left lower lobe collapse and bronchiectasis secondary to bronchiolitis obliterans following an adenovirus infection. In RSV infection, the lungs are often quite clear, or focal areas of superimposed atelectasis may be noted (see Fig. 54-1); when present, these predict a need for more prolonged mechanical ventilation.40 In mild influenza infection, initial chest radiographs are normal or may demonstrate nonspecific prominence of peribronchial markings and hyperinflation. In children with a more severe clinical course, bilateral symmetric and multifocal areas of consolidation, often associated with ground-glass opacities, are seen (e-Fig. 54-3).41 e-Figure 54-3 Lethal H1N1 influenza in an 8-month-old boy who presented with shortness of breath and fever during an outbreak of epidemic flu. The radiographic manifestations of SARS are typically mild, with interstitial thickening, which may progress to focal consolidation.42,43 In children, unlike in adults, no lymphadenopathy, pleural effusion, or cavitation is reported.44 However, abnormalities may persist on thin-section CT in older children for up to 12 months following infection.45 Measles virus is thought to be the cause of giant cell pneumonia, which produces a diffuse reticulonodular bronchopneumonia-like radiographic pattern (e-Fig. 54-4), with hilar node enlargement and superimposed bacterial infection, usually affecting the lower lobes. Atypical measles pneumonia following vaccination with, or exposure to, live virus was characterized by extensive nonsegmental parenchymal consolidation, with pulmonary nodules, hilar lymph node enlargement, and pleural effusion being common. e-Figure 54-4 A 12-year-old girl with leukemia and measles pneumonia. Adenovirus may cause necrotizing bronchopneumonia, bronchitis, or bronchiolitis. Radiographic features are nonspecific but usually include bronchial wall thickening, peribronchiolar densities, air trapping, and patchy or confluent consolidations.30 Adenopathy is more common than in other viral pneumonias. Bronchiectasis or bronchiolitis obliterans may be a permanent sequel (see e-Fig. 54-2).30,31,46 In children infected with CMV, often, a progressive interstitial pneumonitis is seen (e-Fig. 54-5). Gallium-67 scintigraphy may show abnormal uptake in the lungs of patients who have a normal chest CT.47 e-Figure 54-5 Cytomegalic virus infection in a 14-year-old boy with acute lymphoblastic leukemia and immunodeficiency, presenting with dyspnea, fever, cough, and decreased white blood cell counts. Findings in varicella are similar to those of measles pneumonia. Multiple focal calcifications frequently develop after severe chickenpox pneumonia (e-Fig. 54-6). e-Figure 54-6 Chickenpox pneumonia in a teenager. In EBV infection, hilar and mediastinal lymph node enlargement may be seen (e-Fig. 54-7). Pulmonary involvement is uncommon but is characterized by bilateral reticular perihilar infitrates.48 e-Figure 54-7 Mononucleosis in a teenager with hepatosplenomegaly and lymphadenopathy. Treatment and Follow-up: Effective antiviral therapies have not been established, and treatment of viral pulmonary infections is mainly supportive. Experimental treatments to correct the acquired surfactant deficiency and dysfunction that occurs in critically ill infants with viral pulmonary infections are actively being investigated.37 With a few exceptions, the use of extracorporeal membrane oxygenation to treat severe respiratory failure from ARDS, which frequently complicates severe infections, has not lead to better outcomes than that of optimal less invasive supportive care.37 Certain high-risk infants may qualify for prophylactic injection of monoclonal antibodies against the F-glycoprotein of RSV. Since the most important complication of viral pulmonary infection is a superimposed bacterial infection, this should be actively looked for and treated with antibiotics when confirmed.2 Bacterial pulmonary infections are acquired through inhalation, hematogenously, or rarely by direct extension of chest wall or extrathoracic sites. Their course is determined by the balance between the virulence of the organism and the host immune response (mainly humoral, B-cell–mediated immunity, and macrophageal activity). They are characterized by alveolar airspace consolidation, visible as one or more focal lung opacities that exhibit air bronchograms and obliterate normal air–soft tissue interfaces (the silhouette sign). Pleural effusions are common. The most common bacteria and bacteria-like agents causing pulmonary infections in children are listed in Table 54-2. In young children, the classic segmental or lobar airspace consolidation is rarely present, and it is nearly absent in neonates. This is reflected by the reported low 30% positive predictive value of radiographic criteria for a bacterial cause of pneumonia, accounting for a widespread overprescription of antibiotics and development of antibiotic resistant bacteria.1,21 On the other hand, the high 92% negative predictive value of radiographic criteria for bacterial pneumonia is helpful, allowing clinicians to withhold antibiotics in symptomatic children with a negative chest radiograph and to focus on other potential sources of the fever.1,21 It is, therefore, important not to overcall pediatric chest radiographs for the presence of a pulmonary infection, which is the most common interpretation error made by radiologists unfamiliar with pediatric imaging.49,50 Clinically occult pneumonia may be diagnosed with radiography in up to 19% of children less than 5 years old with fever of unknown origin and leucocytosis, although a more recent study found a lower incidence (5.3%) and a low utility of radiography when cough was not one of the presenting symptoms in this setting.51,52 Etiology: This organism is the most common cause of bacterial pneumonia in children less than 5 years of age. It is a gram-positive diplococcus, which infects healthy patients but also commonly attacks those with underlying illness, including the hospitalized and immunocompromised, and children with sickle cell anemia.53 A strong association with a preceding viral infection, in particular influenza, is seen.54 The virally activated respiratory epithelium has an increased expression of receptors for pneumococcal attachment.55 In the usual case of an infected but otherwise healthy child, the onset is acute with fever, headache, and abdominal or chest pain. The pulse and respirations are rapid. By the second day, cough, expiratory grunts, rales, and pleural friction rub may be heard. Rapid clinical resolution usually occurs within 24 to 48 hours after treatment with antibiotics in patients with uncomplicated infections. However, a rapid increase has been seen in the incidence of partially or fully penicillin-resistant strains of S. pneumoniae.56 Following the institution of conjugate pneumococcal vaccination, the proportion of children younger than 5 years with suspected occult pneumococcal pneumonia confirmed by radiography decreased from 15% to 9%.57 Imaging: Pneumococcal pneumonia is usually confined to one lobe, but only rarely is the entire lobe consolidated. A pattern of homogeneous airspace consolidation is usual but not invariable, especially in the presence of underlying lung disease. This pneumonia may initially have a strikingly round appearance in children younger than 8 years (Fig. 54-8), simulating an intrapulmonary mass or abscess, until it spreads further to reach a normal anatomic boundary such as a fissure.1,58,59 Pleural effusion, empyema (e-Fig. 54-9), and lung necrosis (e-Fig. 54-10) are infrequent complications, seen in less than 30% of patients. Resolution on radiographs is usually complete by 6 to 8 weeks. Figure 54-8 Streptococcal round pneumonia in a 5.5-year-old girl presenting with fever and left-sided back and rib pain. e-Figure 54-9 Streptococcal pneumonia and empyema in a 7-year-old girl who presented with high fever, chest pain, cough, shortness of breath and elevated white blood cell count. e-Figure 54-10 Necrotizing pneumococcal pneumonia in a 2-year-old boy who presented with cough and fever. Etiology: Group A streptococcus usually produces tonsillitis or pharyngitis. In the 1990s, S. pyogenes pneumonia, often associated with the toxic shock syndrome, was increasingly reported in childhood. It may occur de novo in a healthy child or follow a viral infection. In neonates, group B streptococcus is a leading cause of sepsis, including pneumonia and meningitis. Imaging: Group A Streptococcus produces a bronchopneumonia in a segmental configuration with either homogeneous or patchy consolidation, which frequently affects a lower lobe. It may be bilateral. Pleural effusion and empyema are common in untreated cases. Lung abscess may be a complication. Clinically and on imaging studies, this pneumonia is very similar to staphylococcal pneumonia, although pneumatoceles are less commonly seen. Group B Streptococcus may accompany or mimic hyaline membrane disease in neonates, and is radiographically difficult to distinguish from it, although the presence of pleural effusion favors (concomitant) infection. Etiology: This gram-positive, catalase-positive coccus primarily affects infants under the age of 1 year (70%). In debilitated patients, it occurs as a superinfection, particularly in the hospital. The incidence of “primary” staphylococcal pneumonia has decreased markedly since the early 1950s. However, staphylococcal pneumonia secondary to septicemia rather than inhalation of organisms is increasing and occurs in older children. This form of “embolic” disease may present with multiple nodular masses or abscesses (e-Fig. 54-11). This evolving pulmonary pattern in a child with sepsis should initiate a search for a distant source of infection, often in bones, joints, or skin.60 Despite extensive pulmonary disease, if recovery occurs it is usually without sequelae. In comparison with methicillin-sensitive strains, methicillin-resistant strains of community-acquired S. aureus cause more serious pneumonias in younger children.61,62 e-Figure 54-11 Embolic form of staphylococcal pneumonia in a 9-year-old boy who presented with a large abscess in the soft tissues of the pelvis. Imaging: In contrast to pneumococcal pneumonia, staphylococcal pneumonia is a lobular or bronchopneumonia, which begins in the airways rather than in the alveoli. Consolidation of peribronchiolar acinar units occurs initially in a segmental distribution. This infectious agent is very virulent, and severe hemorrhagic pulmonary edema may develop rapidly. Pneumatoceles are more common than in any other type (also reported in S. pneumoniae, H. influenzae, and Escherichia coli pneumonia) and occur in 40% to 60% of patients.61,63,64 They usually appear during the first week of the pneumonia and resolve within 3 months. Pneumatoceles often appear as the child is getting better, and their presence does not have prognostic significance. Ten percent of children with staphylococcal pneumonia have a pneumothorax, which may result from the rupture of a pneumatocele.61,63,64 Pleural effusion and empyema are also very frequent, occurring in more than 90% of children (Fig. 54-12).64 Figure 54-12 Role of cross-sectional imaging in a 7-month-old girl with staphylococcal pneumonia and empyema. Etiology: Haemophilus influenzae is a gram-negative, rod-shaped bacterium, which was first discovered in 1892 during an influenza pandemic. In infants and young children, it causes bacteremia, pneumonia, cellulitis, epiglottitis, and meningitis. Since 1990, vaccination has markedly reduced the incidence of this pneumonia (<95%). Imaging: The radiographic appearance is nonspecific: pulmonary opacities that often begin as a segmental, interstitial-appearing process progresses to airspace consolidation.65 Approximately two thirds of cases have unilateral involvement, but more than one lobe is involved 25% of the time.65 Empyema is a common complication, occurring in about 40%.65 Etiology: Bordetella pertussis is a gram-negative, aerobic, capsulated coccobacillus. The incidence of pertussis (whooping cough) has decreased significantly with immunization, but it is still seen in young infants, particularly in the unimmunized. This agent is spread by airborne droplets. A characteristic clinical sign is the paroxysmal cough (whoop). Even after convalescence, the patient’s cough may persist for weeks or months. In China, the disease has been termed “the cough of 100 days.” Imaging: Abnormal but nonspecific findings are present in most patients with pertussis. Because this is an airways-centered disease, it may mimic viral airways disease and pneumonia. The classic appearance is that of the “shaggy heart” (e-Fig. 54-13). However, nonspecific findings such as hyperaeration, atelectasis, segmental consolidations, and hilar lymphadenopathy are seen more commonly. Radiographic changes may persist for several weeks. Bronchiectasis may be a long-term complication. e-Figure 54-13 Pertussis bronchopneumonia. Etiology: Pseudomonas aeruginosa is a gram-negative, aerobic, rod-shaped bacterium, which is an opportunistic pathogen in humans. Lungs, urinary tract, and kidneys are the common sites of infection. P. aeruginosa usually occurs as a nosocomial infection and is a major problem for patients with cystic fibrosis, in which thick layers of lung mucus and alginate produced by the bacteria may limit the diffusion of oxygen. Diagnosis of this infection depends on the Gram stain of sputum or other bacteriologic specimens (e.g., lung tissue, bronchoscopic aspiration, and bronchoalveolar lavage fluid). Imaging: When the patient is infected by airway contamination, the process tends to involve both lung bases, with extensive bilateral parenchymal consolidation, patchy areas of disease with small abscess formation, or small regions of lobular emphysema. In the bacteremic form, widespread patchy or nodular shadows may be found throughout both lungs. Lung necrosis may also occur. Etiology: Anaerobic bacteria are uncommonly responsible for pneumonia and lung abscess.68 Aspiration is the most common route of exposure, and Fusobacterium species, Bacteroides species, Peptococcus, and Peptostreptococcus may be cultured from the abscess or the pleural fluid. Imaging: Usually, consolidation occurs in the lower respiratory tract, and clinical course is slowly progressive (e-Fig. 54-14). Lung abscess (e-Fig. 54-15) and fulminant necrotizing pneumonia may eventually develop. e-Figure 54-14 Anaerobic pulmonary infection in a 15-year-old boy who presented with chronic cough and chest pain without fever. e-Figure 54-15 Anaerobic pulmonary infection in a 12-year-old boy with Wiskott-Aldrich syndrome presenting with multiple cerebral abscesses, septicemia, cough, and fever. Chlamydophila trachomatis and pneumoniae Etiology: Chlamydia trachomatis is an intracellular bacterium commonly found in the genital tract, where it causes urethritis in men and cervicitis in women. In neonates, it causes conjunctivitis, which is contracted during passage through an infected birth canal. Chlamydia is a common cause of pneumonia in infants between 2 and 14 weeks of age.69 The infant is affected by a staccato-like cough and may have conjunctivitis and eosinophilia, although these are not invariable findings. Usually, the patient is afebrile, with a radiographic appearance suggesting an illness more severe than the clinical findings indicate. Recently, C. pneumoniae has been recognized as a common agent causing community-acquired bronchitis and mild atypical pneumonia in school-age children.3 The clinical response to appropriate therapy is usually rapid. Imaging: The radiographic findings of C. trachomatis infections are nonspecific (e-Fig. 54-16), but when they are analyzed in conjunction with clinical findings, the disease may be suspected. Bilateral involvement is usual.70 The lungs are usually hyperaerated with increased linear density and patchy areas of consolidation, probably representing subsegmental atelectasis. Lobar consolidation is rare.70 e-Figure 54-16 Chlamydial pneumonia in a 6-week-old boy with wheezing an a low-grade fever.
Pulmonary Infection
Pulmonary Infections Caused by Viruses
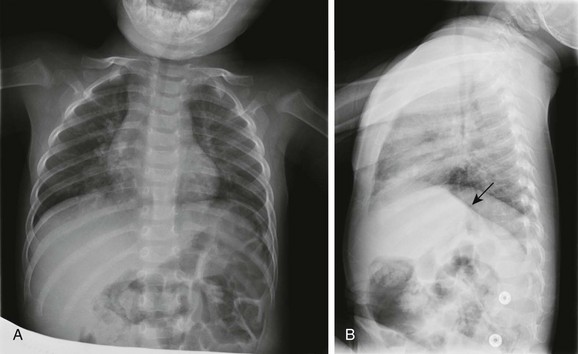
A, The frontal chest radiograph shows perihilar streaky lung opacities and peribronchial thickening, typical of viral infections, with more focal opacity medially in the right lung base, from superimposed atelectasis. This was mistaken for alveolar consolidation indicative of bacterial pneumonia, and antibiotic treatment was given unnecessarily. B, The lateral chest radiograph better demonstrates air trapping in the right lung base, with flattening of the right hemidiaphragm (arrow).
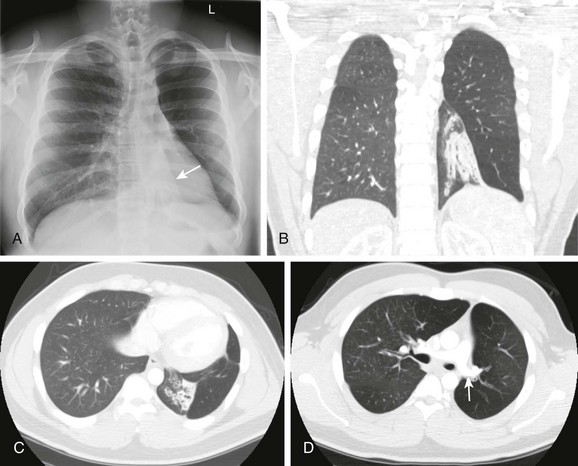
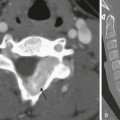
A, The radiograph shows left lower lobe collapse (arrow) and hyperlucency of the left upper lobe. B to D, Computed tomography images demonstrate cylindrical bronchiectasis in the collapsed left lower lobe, and a relatively small left pulmonary artery (arrow in D).
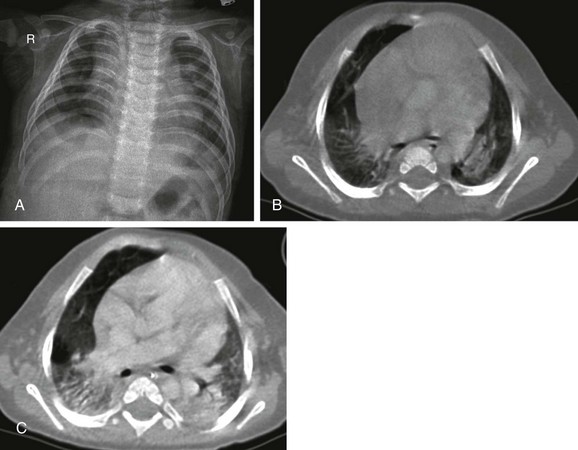
A, The radiograph shows nonspecific increased perivascular markings and somewhat low lung volumes. B, The contrast-enhanced computed tomography (CT) scan demonstrates consolidation in both lower lobes. C, The follow-up CT scan after 1 month showed progression of the consolidations. Soon after this scan was obtained, the baby died from cardiopulmonary failure.
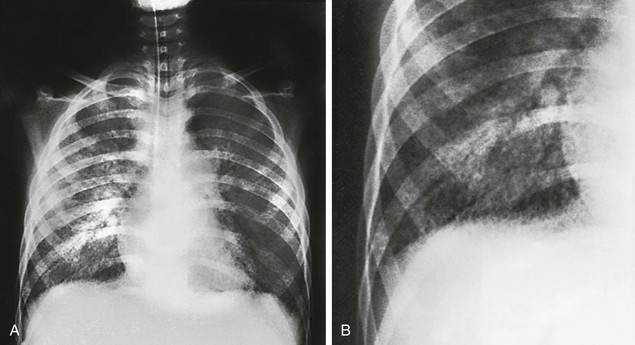
A, Initial radiographic examination showing a diffuse bilateral reticulonodular infiltrate, reflecting the interstitial distribution of the disease. B, Close-up of the right lower lobe showing the reticulonodular pattern to better advantage.
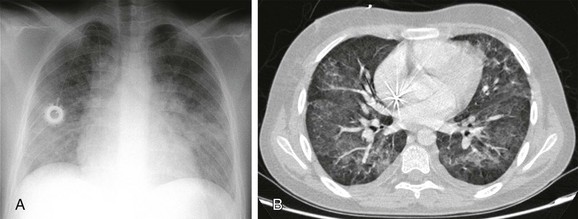
A, The radiograph shows bilateral ill-defined pulmonary vasculature, more prominent in the lower lung zones. B, The contrast-enhanced computed tomography scan demonstrates bilateral ground-glass opacities with relative sparing of the peripheral parenchyma.
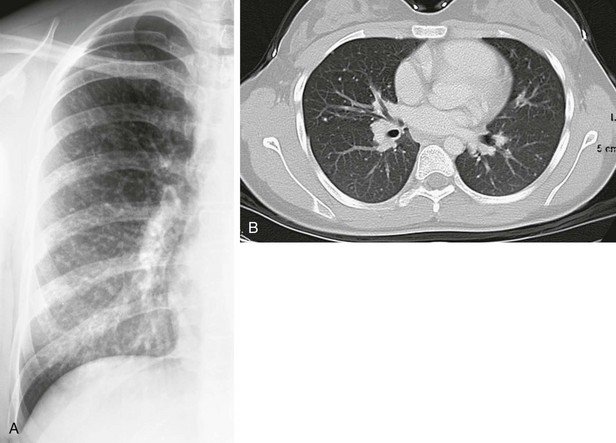
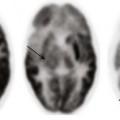
A, The detail from a frontal chest radiograph early in the disease reveals diffuse nodules throughout the right lung. B, A computed tomography scan done for clarification reveals nodules ranging from 2 to 4 mm, and many are densely calcified.
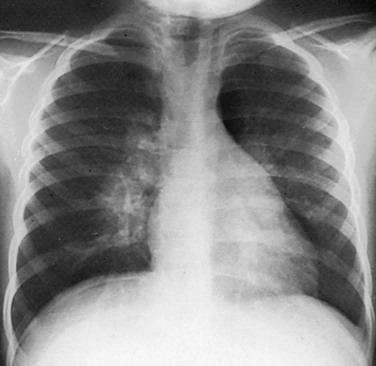
The chest radiograph reveals marked bilateral hilar adenopathy. Lymphoma was considered, but infectious mononucleosis was diagnosed. (Courtesy Dr. Jack Sty, Milwaukee, WI.)
Pulmonary Infections Caused by Bacteria and Bacteria-Like Organisms
Aerobic and Facultative Organisms
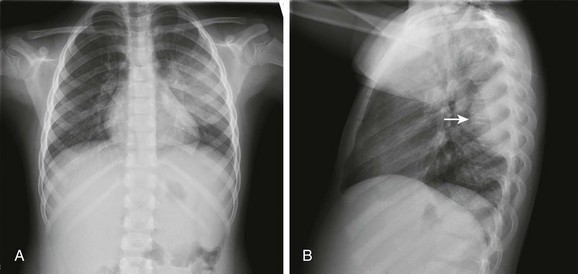
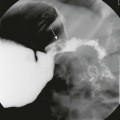
Frontal (A) and lateral (B) radiographs show alveolar consolidation in the superior segment of the left lower lobe with a rounded appearance (arrow in B). Note in A the visibility of the left hilar shadow, which is superimposed on the posteriorly located pulmonary pseudomass (hilar overlay sign).
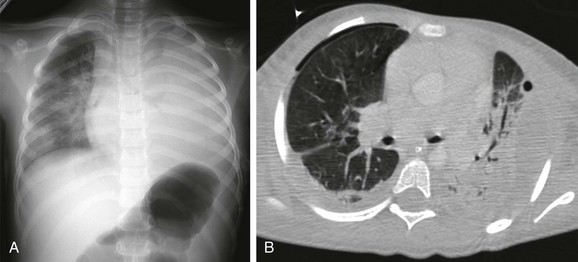
A, The radiograph shows opaque left hemithorax. B, Contrast-enhanced computed tomography demonstrates diffuse consolidation in the left lung and pleural effusion compressing the underlying lung. Air is present in both pleural spaces secondary to intervention. Pleural fluid culture was consistent with Streptococcus pneumoniae type I infection.
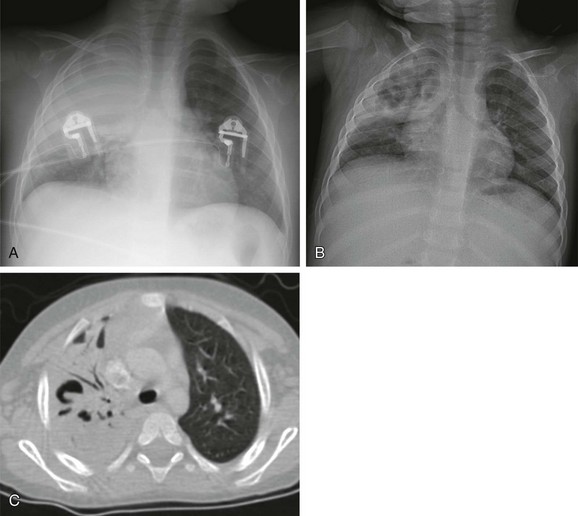
A, The initial radiograph shows lobar consolidation with expansion of the right upper zone. B, On follow-up radiograph in the first week, the consolidation is filled with air cavities, consistent with necrosis. C, Contrast-enhanced computed tomography demonstrates air bronchograms and pneumatoceles in the right upper lobe. Blood culture yielded Streptococcus pneumoniae.
Streptococcus Pyogenes (Group A Streptococci) and Agalactiae (Group B Streptococci)
Staphylococcus aureus
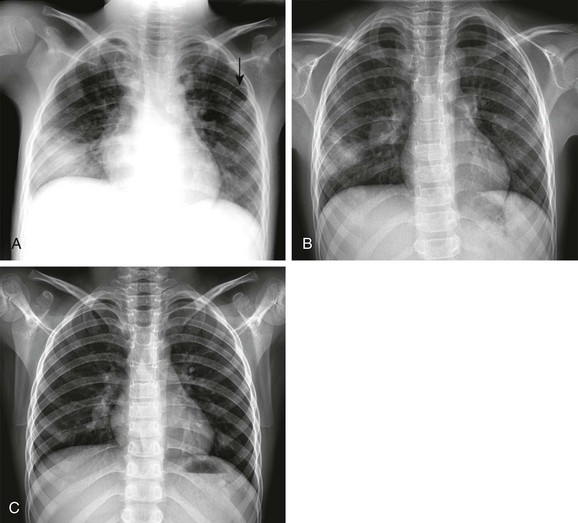
A, The initial radiograph demonstrates a round consolidation in the right lower lung zone and multiple patchy and nodular opacities throughout the lungs. Pneumatoceles are present in both upper lung zones (arrow). B, The follow-up radiograph after 6 days shows marked resolution of the consolidations and pneumatoceles. C, The radiograph on the 30th day shows almost complete resolution of the lesions. Abscess and blood culture yielded Staphylococcus aureus.
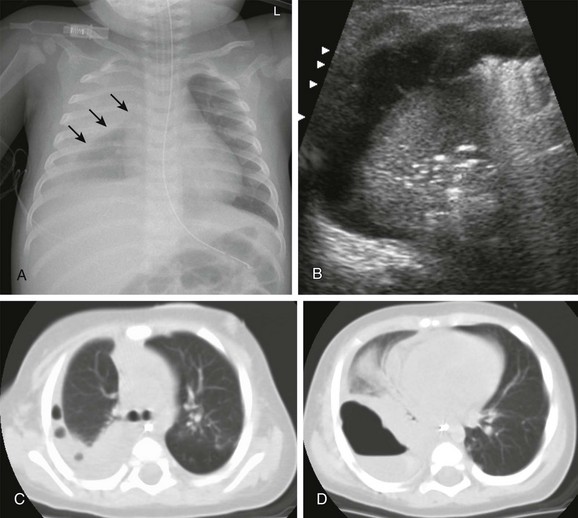
A, The radiograph demonstrates right pleural thickening, which has a convex margin toward the lung (arrows). B, The transverse ultrasound image of the right chest shows a complex pleural effusion, containing thin septations. C and D, Computed tomography confirmed the presence of loculated pleural fluid collections, containing trapped gas bubbles (C) and, more inferiorly, an air-fluid level (D). (Reprinted with permission granted by Springer-Verlag of Westra SJ, Choy G. What imaging should be performed for the diagnosis and management of pulmonary infections?. Pediatr Radiol. 2009;39[suppl 2]:S178-S183 [Figure 1, page S179]).
Haemophilus Influenzae Type B
Bordetella pertussis
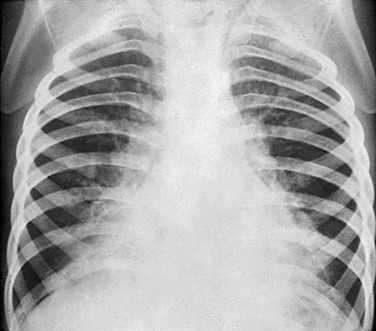
Bilateral basilar bronchopneumonia during the active phase of whooping cough. The heart contours are irregular (“shaggy heart”).
Pseudomonas aeruginosa
Anaerobic Organisms
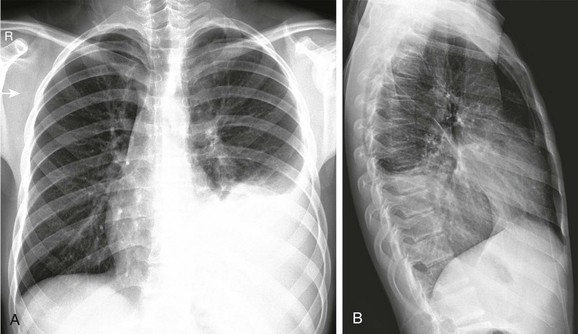
Frontal (A) and lateral (B) radiographs show a large opacity in the left lower thorax that obscures the diaphragm, consistent with consolidation with pleural fluid. Polymerase chain reaction on the drainage fluid from the pleural cavity was consistent with Fusobacterium infection.
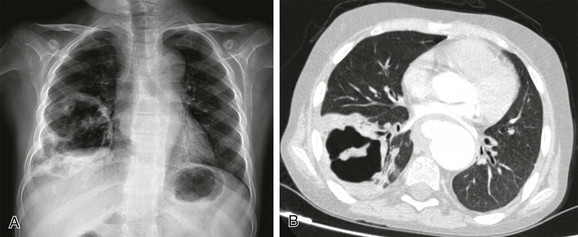
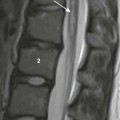
A, The radiograph shows a thick-walled air cavity in the right lower lung zone. B, Contrast-enhanced computed tomography depicts the pulmonary abscess. There is also a pseudoaneurysm in the descending thoracic aorta. Polymerase chain reaction test of the cerebral abscess was consistent with Peptostreptococcus infection.
Bacteria-Like Organisms
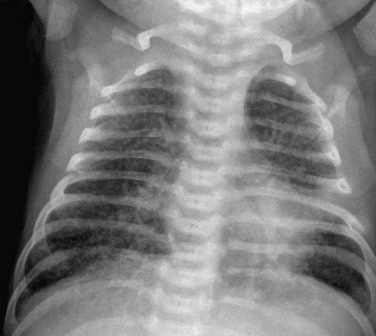
The frontal radiograph shows diffuse interstitial infiltrates with hyperinflation. Polymerase chain reaction results confirmed the diagnosis.