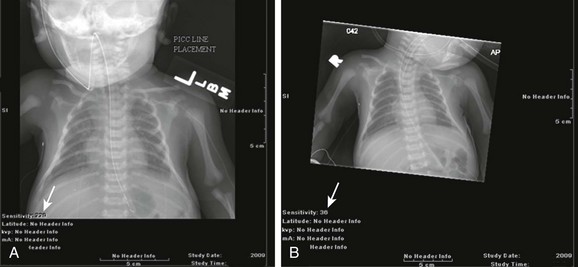
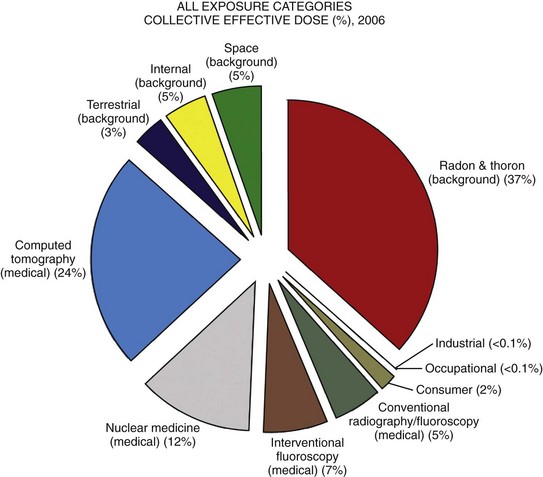
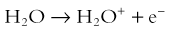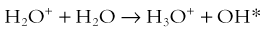Chapter 1 Diagnostic imaging has evolved from the single technique of radiography discussed in the first edition of Caffey’s Pediatric X-Ray Diagnosis in 1945 to a specialty with a choice of many modalities and techniques. Many of these modalities use ionizing radiation, and some entail relatively high doses of radiation, such as computed tomography (CT) and nuclear imaging, including positron emission tomography.1 For this reason, the imaging community (and our medical colleagues) must jointly adhere to two of the principles of radiation protection for our patients: justification (i.e., the examination is appropriate) and optimization (i.e., the appropriate technique is used). For example, we can substantially influence the radiation dose range within the radiographic arena depending on how we perform computed or direct digital radiographic examinations. Image processing for this digital technology can accommodate overexposures; that is, from a contrast and brightness standpoint, the image can be adjusted to appear as if it was obtained using standard techniques, whereas with screen film technology, the film image would be recognized as overexposed (dark) (Fig. 1-1). In addition, without accountability for displaying dose metrics (such as the exposure index available with digital radiography), it is difficult or impossible to account for patient exposures in clinical practice.2 In addition, uninformed and potentially irresponsible justification of medical imaging occurs when persons are unfamiliar with the methods of estimating an effective radiation dose during CT examinations in children.3,4 Increasing accountability is expected from the medical community with regard to the use of imaging modalities that expose children (as well as adults) to ionizing radiation, especially for the risk part of the risk/benefit ratio. Because of the need for such accountability, a basic understanding of radiation biology, including bioeffects, radiation doses of various types of imaging examinations, and risks of radiation, is essential for the pediatric imager. A glossary of terms and dose descriptors is found in the addendum at the end of this chapter. Approximately 4 billion imaging examinations using ionizing radiation (i.e., radiography, fluoroscopy/angiography, CT, and nuclear imaging) are performed annually worldwide.5 In the United States, medical imaging currently accounts for a significant percentage of the annual radiation exposure to the population (Fig. 1-2),6 and most sources demonstrate continued increased use during the past decade. For example, one study showed a fivefold increase in CT examinations in the pediatric acute care setting from 1995 to 2008.7 Natural or background sources account for about 50% of the annual radiation exposure in the United States, and diagnostic medical radiation accounts for most of the remainder, an approximately sixfold increase during the past 30 years. CT alone accounts for nearly 25% of all radiation exposure to the U.S. population.6 Many reasons exist for the increased use of diagnostic medical radiation, and much use of such radiation is based on sound medical decision making. However, other factors determine use as well, including defensive medicine. Hall8 has written an excellent review of radiobiology for the radiologist. The biologic effects of radiation result primarily from damage to deoxyribonucleic acid (DNA)—the critical target. The first step in the absorption of x-rays is that the x-ray particle, the photon, gives up its energy to produce a fast recoil electron. The electron may damage DNA directly, but the electron also can interact with a molecule of water to produce a free radical (Fig. 1-3). A free radical is a highly reactive atom or molecule with an unpaired electron in the outer shell: Figure 1-3 Direct and indirect action. where the asterisk indicates a free radical. The hydroxyl radical (OH) can diffuse a short distance to cause DNA damage. The fact that two thirds of x-ray damage occurs via OH suggests that someday this component of radiation damage might be reduced through the use of chemical radioprotectants. The topic of radioprotectants was recently well reviewed.9 The biologic effects of radiation result primarily from damage to double-stranded DNA as opposed to single-strand injury (see Fig. 1-3). Single-strand breaks of DNA are readily repaired and are presumed to have a negligible effect. Breaks in both DNA strands that are opposite or separated by a few base pairs are much more difficult to repair. These double-strand breaks can cause important biologic effects, including genetic mutations, carcinogenesis, and cell death. Dicentric and fragmented breaks typically result in cell death, whereas nonlethal translocation repairs may cause impaired cellular function, including development of an oncogene.8 The biochemical and physiologic damage produced by radiation generally occurs within hours or days, but the impact of these changes, such as the induction of cancer, can take decades to manifest. This carcinogenesis process has several steps. Aberrations in chromosomes (e.g., deletions, translocations, or aneuploidy) are produced by DNA damage. Because these impaired cells survive, they become “stable aberrations” (some with neoplastic transformation), a morphologic change that is the first step of a multistep process to radiation-induced carcinogenesis. The second step of the process is cellular immortality. That is, most cancer cells are descendents of a single cell that originally underwent neoplastic transformation. The third step is tumorgenicity. The radiation exposure induces a cellular genomic instability that is transmitted to progeny, which Little10 described as “a persistent enhancement in the rate of which genetic changes arise in the descendents of the irradiated cells after many generations of replication … [this process] has been termed a nontargeted effect of radiation as genomic damage occurs in the cells that in themselves receive no direct radiation exposure.” Most childhood tumors (about 85%) occur sporadically, but in 15% of the cases, a strong family association and genetic basis for radiation sensitivity are present. Persons with certain diseases are uniquely sensitive to radiation-induced cancers, although the exact mechanism is unclear (Box 1-1). The two types of biologic effects from radiation are deterministic and stochastic (random). Deterministic bioeffects are characterized by a threshold dose, and the severity of the effect is dose dependent. For example, cataracts traditionally are reported not to occur with less than a 2.0 Gy exposure, although recent data suggest that this threshold is below 1.0 Gy.11 Table 1-1 shows some of the doses for deterministic effect. In general, deterministic effects from the doses used in diagnostic imaging are extremely rare. Recent exceptions with head perfusion CT scanning in adults have been reported.12 Deterministic effects such as skin ulcers and burns should never occur from diagnostic imaging, but they are seen with relatively lengthy interventional procedures. Table 1-1 Modified from Hall EJ. Radiobiology for the radiologist, ed 5, Philadelphia: Lippincott Williams & Wilkins; 2000. From a public health perspective, all ionizing radiation from medical imaging is considered to be potentially harmful because we assume that no threshold exists below which radiation is safe (i.e., no harmful effects will occur). This “linear no threshold” model is applied to low-level radiation exposure.13,14 The effects of radiation are greatest on rapidly developing organisms—that is, fetuses, infants, and young children. In pregnancy, the major biologic effects of fetal demise, growth restriction, organ malformations, and cognitive deficits are seen only with doses far in excess of routine diagnostic imaging.15 The risk of developing cancer from exposure of a fetus to radiation is uncertain, as it is with exposure of a child to radiation; potential effects could be seen with uterine doses that occur as a result of relatively high direct exposures (e.g., a pelvis CT scan for possible appendicitis). No data in humans indicate that genetic effects result from diagnostic levels of radiation. Compared with middle-aged adults, children have been reported to be from 2 to 15 times more sensitive to radiation-induced carcinogenesis.16,17 However, Shuryak et al18 recently noted that the cancer induction risk (greater at younger ages) must be balanced with the radiation-induced promotion of premalignant damage (greater in middle age), which may differ for certain types of cancer. Thus cancer risks may be higher in the adult population than traditionally believed.18 Low-dose effects have been described by Pierce and Preston,19 who studied the data from atomic bomb survivors reported by the Radiation Effects Research Foundation. Among persons who had received dosages of 0.005 to 0.2 sievert (500 mrad to 20 rad), 35,000 people survived, and 5000 cases of cancer developed. The authors made the following conclusions: first, the solid cancer risk persists for more than 50 years. Second, there is a 10% increase over the expected cancer rate.19 Low-dose relative risk factors are shown in Figure 1-4. Figure 1-4 Estimated low-dose risks. The overall risk of cancer for the entire population suggested by the International Commission on Radiological Protection is 5% per sievert for low doses and low-dose rate. However, this value is an average value. For adults in late middle age, the risk decreases to only 1% per sievert, whereas for children in the first decade of life, the risk may be as high as 16% per sievert for girls and 12% per sievert for boys. The female dose is higher because of the greater incidence of breast and thyroid cancers (Fig. 1-5). Radiation risks from diagnostic imaging low-level radiation were reviewed recently.20 Two excellent reviews also recently were published by Linet et al21,22 (Table 1-2). At this point, we have cautious uncertainty regarding cancer risk and low-level radiation. As Hricak et al23
Radiation Bioeffects, Risks, and Radiation Protection in Medical Imaging in Children
Trends in Medical Radiation Exposure to Children
Pathophysiology of Radiation Effects
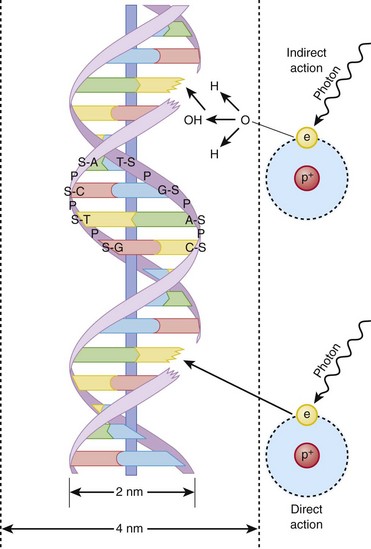
Indirect action (top part of figure), an electron damages the deoxyribonucleic acid (DNA). In indirect action, the secondary electron interacts with a water molecule to produce a hydroxyl radical that then damages DNA, in this case affecting a single strand. (From Hall EJ. Radiation biology for pediatric radiologists, Pediatr Radiol 39(1):S57-S64, 2009. Used with permission.)
Types of Radiation Bioeffects
Fetuses and Children Have Greater Radiation Risks
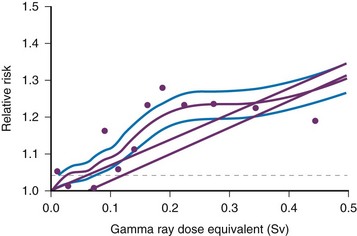
Age-specific cancer rates over the 1958 through 1994 follow-up period relative to the rates for unexposed people averaged over the follow-up for sex and for age at exposure. The dashed line represents ±1 standard deviation error for the smooth purple curve. The upper straight line is the linear risk estimate computed from the range 0 to 2 Sv. The second straight line beginning at 0.06 Sv is the upper 95% confidence limit for such a quantity. (From Pierce DA, Preston DL. Radiation-related cancer risks at low doses among atomic bomb survivors, Radiat Res. 154:178-186, 2000.)
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiology Key
Fastest Radiology Insight Engine