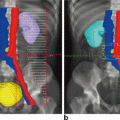
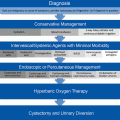
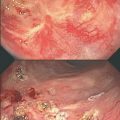
Fig. 7.1
a and b Cystoscopic view of radiation cystitis
Normal bladder urothelium consists of several layers of polyhedral (transitional) cells divided into three main layers from surface to base: umbrella, intermediate, and basal cells. Basal cells divide to form intermediate cells and intermediate cells fuse to form umbrella cells. Under normal conditions, the rate of turnover of urothelial cells is very slow ranging anywhere from 6 weeks to 1 year in the mouse bladder [5, 6]. The urothelium acts as a protective barrier that allows urine to be stored for a longer time while maintaining initial concentration.
The acute effects of radiation on urothelium are characterized by mucosal edema, hyperemia, and inflammation. Using measurements of urinary frequency and cystometry, Stewart et al. noticed that the acute phase of radiation-induced damage manifests as a dose-dependant increase in urinary frequency. Data on cellular and molecular mechanisms underlying radiation effects in the murine urinary bladder suggest that the acute effects are associated with changes in urothelial protein expression such as Uroplakin III, CD 18, CD 44, or syndecan [7]. It is hypothesized that these proteins maintain the integrity of the normal urothelial barrier to urine. Altered expression of these proteins leads to disruption of the urothelial barrier with increased exposure of underlying tissue to urine and its toxic solutes contributing to irritative voiding symptoms. Experimental studies also indicate that altered prostaglandin metabolism, with increased cyclooxygenase-II expression which regulates the tone of the bladder wall, results in increased baseline tone of the detrusor muscle corresponding to a reduction in bladder capacity. [8]. Unlike other epithelia, urinary epithelial cell depletion is not noted in the acute phase of radiation damage due to the very long turnover time of the urothelium. However, the presence of infection may complicate this early response, which may then progress to desquamation and ulceration.
Late damage is irreversible and is characterized by epithelial denudation, and ulceration with focal hyperplasia and fibrosis [9]. Kraft et al. showed that transforming growth factor-β (TGF-β) is overexpressed in the mouse urinary bladder between days 90 and 360 after radiation [10]. The increased expression was associated with increased collagen deposition in the bladder wall; however, these changes did not correlate with the functional radiation response, and hence are considered a secondary effect to organ damage rather than a primary radiation effect. In contrast, a clear correlation was noted between changes in bladder function and urothelial changes. The latter presents as focal urothelial denudation and a hyperproliferative response [10]. Jaal et al. noted that these late effects appear to be correlated with increased urothelial expression of intercellular adhesin molecule 1 (ICAM-1) [11].
Radiation-induced damage is also observed in the bladder vasculature. Edema of the vascular endothelium is noted by approximately 3 months following radiation. By 6 months to 2 years, endothelial cell proliferation, perivascular fibrosis, and vascular occlusion occur. Focal bladder wall ischemia leading to bladder wall fibrosis can be seen in severe cases [12]. The loss of balance between endothelial cell proliferation and small vessel maturation is believed to cause development of telangiectasia [13]. These thin-walled tortuous abnormally dilated vessels are prone to rupture and bleeding, resulting in microscopic or gross hematuria. Radiation effect is also noted in the smooth muscle layer of the bladder in the form of loss of smooth muscle, infiltration of fibroblasts, and increase in collagen deposition contributing to loss of bladder compliance and capacity [14].
Clinical Manifestation of Radiation Bladder Injury
The clinical manifestations of radiation injury to the bladder can be classified into acute and late reactions. Acute reactions occur within 3 months after radiation exposure and subside within several weeks after radiation therapy. Late reactions are those that occur at least 3 months after radiation exposure.
Acute Toxicity
Acute lower urinary tract symptoms due to radiation can be irritative or obstructive. Irritative symptoms include urinary frequency, urgency, dysuria, and nocturia. Obstructive symptoms include weak urinary stream, hesitancy, and incomplete bladder emptying or complete bladder outlet obstruction with overflow incontinence. The reported incidence of acute radiation toxicity varies from 20 to 80 % [15–17]. Such wide range in incidence rates reflects the differences in treatment techniques, radiation dose, and treatment fields for various pelvic malignancies. Due to the frequency of such symptoms and the fact that most subside with conservative measures, they are not usually reported as complications but regarded as acceptable outcome to RT. The Radiation Therapy Oncology Group (RTOG) has defined scoring criteria for qualitative assessment of the degree of acute radiation morbidity (Table 7.2). [18] Acute toxicity is scored from day 1 to 90.
Late Toxicity
Late effects from pelvic RT are the result of bladder fibrosis and microvascular alterations resulting in decreased bladder capacity and fragile bladder mucosa. In contrast to acute toxicity, late manifestations tend to be chronic and irreversible. Depending on the dose and treatment plan of the pelvic radiation, late toxicity can involve the entire bladder or a portion of bladder. In general, the time to onset of late toxicity typically is 2–3 years after treatment. Patients with a severely contracted bladder present with debilitating urinary frequency and incontinence. Another chronic manifestation of pelvic RT is late recurrent hematuria or hemorrhagic cystitis, defined as acute or insidious diffuse vesical bleeding that can sometimes be life threatening. It is important to distinguish the side effects of radiation from the effects of underlying disease as well as from the effects of previous pelvic surgery and chemotherapy. It is also important to rule out secondary malignancies such as urothelial carcinoma or recurrence of the primary tumor. The RTOG has published criteria to grade late effects of radiation to the bladder; noting that late effects tend to accrue with time and that long-term follow-up is necessary to accurately assess the late effects of radiation (Table 7.1) [18].
Grade | RTOG-acute | RTOG-chronic |
|---|---|---|
0 | No change | None |
1 | Frequency of urination or nocturia twice pretreatment habit/dysuria, urgency not requiring medication | Slight epithelial atrophy; minor telangiectasia (microscopic hematuria) |
2 | Frequency of urination or nocturia that is less frequent than every hour. Dysuria, urgency, bladder spasm requiring local anesthetic (e.g., Phenazopyridine hydrochloride) | Moderate frequency and dysuria; generalized telangiectasia; intermittent macroscopic hematuria |
3 | Frequency with urgency and nocturia hourly or more frequently/dysuria, pelvis pain, or bladder spasm requiring regular, frequent narcotic/gross hematuria with/without clot passage | Severe frequency and dysuria; severe telangiectasia; frequent hematuria; reduction in bladder capacity (< 150 cc) |
4 | Hematuria requiring transfusion/acute bladder obstruction not secondary to clot passage, ulceration, or necrosis | Necrosis/contracted bladder (< 100 cc) |
5 | Death |
Table 7.2
CTCAE (Cancer Therapy Evaluation Program) version 4.0: A systematic grading system for adverse events of cancer therapy [19]
Grade | CTCAE |
|---|---|
0 | No change |
1 | Asymptomatic or mild symptoms; clinical or diagnostic observation only; intervention not indicated |
2 | Moderate, local, or noninvasive intervention indicated; limiting instrumental activities of daily living (ADL) |
3 | Severe or medically significant but not immediately life-threatening; hospitalization or prolongation of existing hospitalization indicated; disabling; limiting self-care ADL |
4 | Life-threatening consequences; urgent intervention indicated |
5 | Death |
Scoring Systems
Several validated grading systems have been proposed to rate the bladder toxicity secondary to radiation. The main use of these scoring systems is to achieve standardization among different studies on this subject. In 1995, working groups of the RTOG and European Organization for Research and Treatment of Cancer (EORTC) developed the Late Effects in Normal Tissue subjective, objective, management and analytic (LENT-SOMA) scales in an attempt to provide a comprehensive system for assessment and recording of RT-related morbidity [18] (Table 7.1). In 2003, the National Cancer Institute (NCI) published the Common Terminology Criteria for Adverse Events (CTCAE) [19], which incorporated the LENT-SOMA items with early and late effects contained in one system (Table 7.2). An RTOG grading system specifically addressing the severity of hematuria has also been proposed (Table 7.3) [20]. This is now seen as the preferred platform for documenting toxicity in clinical trials. In addition, the American Urological Association’s symptom score can be used to grade the severity of urinary symptoms in these patients. Scores of 0–7, 8–19, and 20–35 signify mild, moderate, and severe symptoms, respectively [21].
Grade | Acute hemorrhagic radiation cystitis (RTOG scale) | Late hemorrhagic radiation cystitis (RTOG/EORTC scale) |
|---|---|---|
1 | NA | Minor telangiectasia (microscopic hematuria) |
2 | NA | Generalized telangiectasia (macroscopic hematuria) |
3 | Gross hematuria with or without clot passage | Severe generalized telangiectasia (macroscopic hematuria)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|