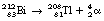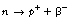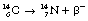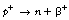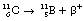Chapter 19 Radioactivity
Chapter contents
19.1 Aim
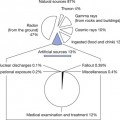
The aim of this chapter is to discuss the various types of radioactive decay which can occur. Within the chapter the relevance of these processes to nuclear medicine will be considered.
19.2 Introduction
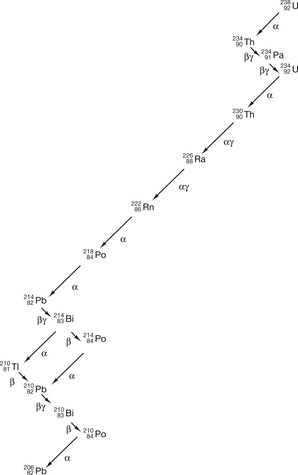
A pictorial representation of the decay process (see Fig. 19.1) is called a decay scheme. In addition to this, it is possible for a nucleus to undergo more than one type of transformation (see Fig. 19.12 later in this chapter) and this type of decay is called a branching scheme.
It is impossible to determine the exact time when a particular nucleus will transform, but the laws of probability may be used to determine the behaviour of a large number of nuclei (see Ch. 20 for a discussion of radioactive decay and the exponential law).
The unit of radioactivity is the becquerel (Bq) where 1 becquerel is 1 nuclear disintegration per second.
19.3 Nuclide chart
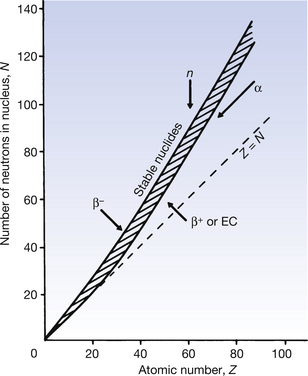
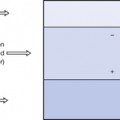
It is often useful to draw a graph, plotting the number of neutrons in a nucleus against the number of protons, as all nuclides may be included in this nuclide chart. Such a chart is shown in simplified form in Figure 19.2. Note that isotopes (lines of equal atomic number) are given by any vertical line on the figure. In such a graph, an angle of 45° to the x-axis represents a situation where the nucleus contains equal numbers of protons and neutrons (Z=N).
It is found on such a graph that there is a broad band of nuclides with low atomic numbers at about 45° to the x-axis and these are all stable or only weakly radioactive. Thus, for the lighter elements, nuclear stability can be produced with equal numbers of protons and neutrons. From the graph it can be seen that, as the atomic number increases, a proportionately larger number of neutrons is necessary to produce nuclear stability. Thus we can say that as the Coulomb repulsion (see Sect. 6.4) between the protons increases, then more neutrons are required to produce the short-range nuclear forces (see Sect. 18.3.1), thus producing the cohesive forces required for a stable nucleus. Nuclei which have atomic numbers greater than 83 (bismuth) are so large that it is impossible to produce a stable nuclear configuration.
Nuclides whose combination of neutrons and protons means that they land outside the band of stability shown in Figure 19.2 have nuclei possessing higher energies than those within the band. As a consequence, such a nucleus is unstable and tends, on decay, to produce a new nucleus of lower energy which is closer to, or within, the stable band. The energy difference between the nucleus before and after decay is emitted either as a charged particle or as a quantum (or quanta) of electromagnetic radiation. The decay scheme towards stability may be in the form of a single step (e.g. 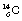 decays to stable
decays to stable 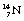 1 by the emission of a beta-particle (see Fig. 19.4) or it may be in the form of a multistage route involving many nuclear transformations (e.g. the decay of
1 by the emission of a beta-particle (see Fig. 19.4) or it may be in the form of a multistage route involving many nuclear transformations (e.g. the decay of 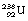 to
to 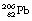 involves at least 14 steps, as shown in Figure 19.1). As a general rule, the greater the mass number of the nuclide, the more complicated the decay path to eventual stability.
involves at least 14 steps, as shown in Figure 19.1). As a general rule, the greater the mass number of the nuclide, the more complicated the decay path to eventual stability.
The effect of different decay modes is illustrated using arrows on Figure 19.2 The direction of the arrow shows the direction of change of position on the chart before and after the decay process. You may find it helpful to refer back to this chart while studying the decay processes in more detail in the following sections of this chapter.
19.4 Alpha decay or alpha-particle emission
For the spontaneous emission of an alpha-particle from a nucleus, the nuclide must have an atomic mass number greater than 150. The nucleus must also have too few neutrons for the number of protons – a higher neutron-to-proton ratio would be required to produce nuclear stability. The alpha-particle consists of two protons and two neutrons tightly bound together (a helium nucleus). It may be considered as a free particle having high kinetic energy which is trapped in the parent nucleus. Thus the daughter nucleus has two protons and two neutrons fewer than the parent nucleus (see Equation 19.1 below).
The mechanism of production of the alpha-particle is quite complex. As we have already identified (see Sect. 18.3.1), the nucleus depends on a balance of disruptive electrostatic (Coulomb) forces and attractive forces between the nucleons caused by the short-range nuclear forces. In very large nuclei there is a large amount of electrostatic repulsion between the protons which extends across the whole nucleus. This is balanced by the short-range nuclear force which exists between adjacent nucleons. Thus, if the nucleus becomes elongated, the electrostatic forces dominate and the nucleus becomes even more elongated. This process continues until the nucleus divides into two fragments, the daughter nuclide and the alpha-particle.
This reaction can also be written as shown in Figure 19.3 (the decay of 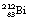 also includes the emission of a beta-particle and is discussed later as an example of a branching programme; for simplicity only the alpha-particle reaction is shown in Fig. 19.3 and the percentages shown refer only to the alpha-particles). Because the daughter product is to the left of the parent nuclide, this shows that there is a reduction in the atomic number. The reaction also shows that the difference in energy between the parent nuclide and the daughter nuclide is 6.08 MeV. If we consider only the alpha-particles emitted, we find that approximately 71% of these have an energy of 6.04 MeV and 29% have an energy of 6.08 MeV. In the case of the first group of alpha-particles, the nucleus is left in an excited state with excess energy of 0.04 MeV which it emits as a gamma-ray.
also includes the emission of a beta-particle and is discussed later as an example of a branching programme; for simplicity only the alpha-particle reaction is shown in Fig. 19.3 and the percentages shown refer only to the alpha-particles). Because the daughter product is to the left of the parent nuclide, this shows that there is a reduction in the atomic number. The reaction also shows that the difference in energy between the parent nuclide and the daughter nuclide is 6.08 MeV. If we consider only the alpha-particles emitted, we find that approximately 71% of these have an energy of 6.04 MeV and 29% have an energy of 6.08 MeV. In the case of the first group of alpha-particles, the nucleus is left in an excited state with excess energy of 0.04 MeV which it emits as a gamma-ray.
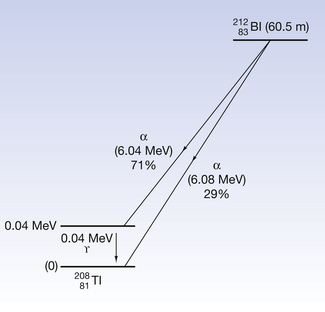
Figure 19.3 An example of alpha-particle emission. The nucleus decreases its atomic number and energy as shown.
19.5 Beta decay or beta-particle emission
In the process of beta decay, a particle, having a mass equal to that of an electron, is ejected from the nucleus. The ejected particles, however, may have either a positive or negative charge and so, although they are known collectively as beta-particles, negative beta-particles (β−) or negatrons and positive beta-particles (β+) or positrons both exist. Although the negatron is exactly the same as an electron, in this and subsequent chapters the term negatron will be used to describe the particle which exits from the nucleus of an atom while the term electron will be used to describe particles which orbit the nucleus of the atom. Because the processes are different for the production of the negatron and the positron, they will be dealt with under separate headings.
19.5.1 Negatron (β−) emission
As we can see from Figure 19.2, β−-particles are emitted from nuclei which have too many neutrons for nuclear stability. As we saw in Chapter 18, nucleons are being constantly changed from proton to neutron and back within the atomic nucleus. A neutron may be thought of as consisting of a proton and a negatron:
This can also be illustrated using the methods discussed for alpha-particle emission to show the transformation from parent to daughter product. Such a diagram is shown in Figure 19.4. Note that this time there is an increase in the atomic number so the line is down and to the right. This simple decay process is an example of pure beta emission since no other transformations are involved.
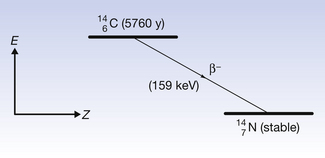
Figure 19.5 shows a more complex emission pattern where the parent and daughter nuclei are separated by an energy difference of 2.81 MeV. The β−-particle has an energy of 0.31 MeV and so the nucleus is left in an excited state – 2.50 MeV above its ground state. The nucleus emits this energy in the form of two gamma-rays, one of energy 1.17 MeV and the other of energy 1.33 MeV. (This will be discussed further when we consider gamma-ray emission in Sect. 19.6.)
19.5.2 Positron (β−) emission
If we consider Figure 19.2, we see that positron emission takes place from nuclei which have too many protons to achieve stability. In such cases, protons appear to become isolated within the nucleus and then to decay to form a neutron which rejoins the nucleus and a positron which is ejected from the nucleus. In this reaction, the atomic mass number will remain the same but the atomic number will decrease by one (the total number of protons and neutrons is unchanged but one proton has been converted into a neutron).
An example of such a reaction is the decay of carbon-11 to boron:
If we consider the energy changes which take place within the nucleus, as shown in Figure 19.6, we see that the situation regarding energy of the β+-particle is not as simple as it is for the β−-particle. The energy difference between the parent and daughter nucleus in this case is 1.99 MeV. An energy loss of 2mc2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree