Fig. 1
Esophageal coin removal in a 2-year-old girl. a Barium esophagram with 14-French Foley catheter demonstrating the esophageal coin (arrow) at the thoracic inlet without complicating food bolus. b Foley catheter balloon inflated, withdrawing the coin into the hypopharynx and mouth for expectoration
Soft-tissue foreign bodies are a common clinical problem in both children and adults (Shiels et al. 1990; Shiels 2007; Close et al. 2009; Young et al. 2010). Most soft-tissue foreign bodies are impaled during domestic activity, involve the superficial soft tissues as a result of low velocity trauma and the majority can be removed without the need for image guidance. High velocity objects embedded in deep soft tissues are usually seen in as a result of weapons, blast injuries, or motorized tool-related accidents.
The primary indications for soft-tissue foreign body removal are recurrent pain or the development of infectious complications (Shiels 2007; Close et al. 2009; Young et al. 2010). With the advent of meticulous sonographic techniques, high-resolution sonography is the main imaging tool used for detection and localization of nonradiopaque soft-tissue foreign bodies, as well as for precise guided removal of radiopaque and nonradiopaque foreign bodies. Sonography is effectively used to guide the administration of percutaneous operative field local anesthesia, hydrodissect soft tissues surrounding the foreign body, blunt dissection, and forceps removal of foreign bodies (Fig. 2). In cases when granulation or fibrotic tissue encases a chronic embedded foreign body, sonography is used to guide sharp dissection (scalpel or large gauge needle [e.g., 12 G angiocatheter needle]) of the foreign body prior to forceps removal (Shiels 2007; Close et al. 2009; Young et al. 2010).
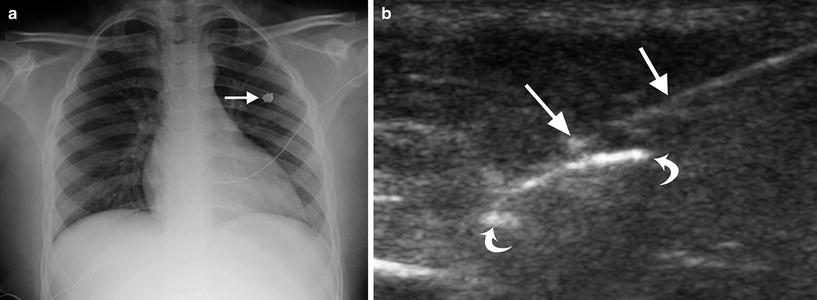

Fig. 2
Percutaneous removal of a chest wall foreign body from a gunshot injury in a 12-year-old male. a Supine AP chest radiograph demonstrating metallic bullet shell fragment (arrow) in the anterior chest wall soft tissues. b Ultrasound showing the metallic shell casing (curved arrows) and forceps (straight arrows) prior to grasping of the foreign body during ultrasound-guided removal
3.2 Esophageal Stricture Balloon Dilation
In children, the most common indications for esophageal stricture balloon dilation are feeding intolerance following surgical repair of esophageal atresia, congenital dystrophic epidermolysis bullosa, and stricture from caustic injestion (Ball et al. 1984; Spiliopoulos et al. 2012; Ko et al. 2006). The barium esophagram in such patients classically demonstrates food pooling in the upper esophageal pouch, a variable-sized anastomotic stricture, and poor motility in the distal esophageal segment. Fluoroscopically guided low-profile angioplasty balloon stricture dilation is most often performed carefully over a series of weeks, progressively dilating the stricture to 10 mm (30F) in young infants (Fig. 3) and 12 mm (36F) in older infants and toddlers (author recommendation, unpublished). Adjunctive topical administration of mitomycin-C during stricture dilation is a promising technique for reducing the rate of stricture recurrence (Chung et al. 2010; Heran et al. 2008).
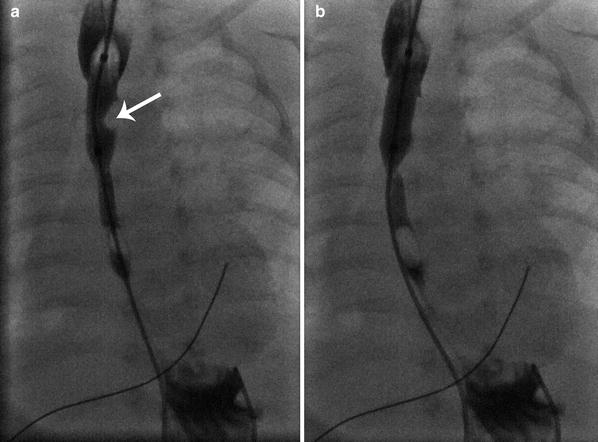

Fig. 3
Balloon dilation of anastomotic stricture in a 1-month-old boy following repair of esophageal atresia. a Fluoroscopic image showing a waist-like impression by the stricture on the 10 mm balloon (arrow) during dilation. b Image following full 10 mm balloon inflation with elimination of the stricture without esophageal rupture
Radiological intervention following surgical treatment of achalasia centers on balloon dilation of post-myotomy strictures. Balloon dilation of a distal esophageal stricture associated with achalasia with fluoroscopic guidance is safe to a diameter of 30 mm.
3.3 Percutaneous Drainage of Parapneumonic Effusion and Treatment of Empyema
Thoracentesis is occasionally performed as an adjunctive procedure in the diagnostic management of children with pneumonia, as a means of isolating organisms, treating associated respiratory distress from a large parapneumonic effusion, or treating pleural empyema. Simple thoracentesis is most safely and efficiently performed using sonographic guidance. Angiocatheter needles of 20–22 gauge provide excellent access systems with polyethylene sheaths that can be used for fluid aspiration without risk of pleural laceration. If a parapneumonic effusion is causing respiratory distress, catheter drainage is preferred over simple thoracentesis followed by immediate removal of the aspiration sheath. Mitri and colleagues demonstrated that simple thoracentesis followed by immediate removal of the aspiration sheath is associated with a 27 % rate of recurrence of the parapneumonic effusion, necessitating a second sedation/anesthesia and drainage catheter placement (Mitri et al. 2002). For this reason, after initial catheter drainage of the parapneumonic effusion, the author and his colleagues routinely maintain the drainage catheter in place for a few days, until pleural drainage ceases during medical treatment of the associated pneumonia (Fig. 4).
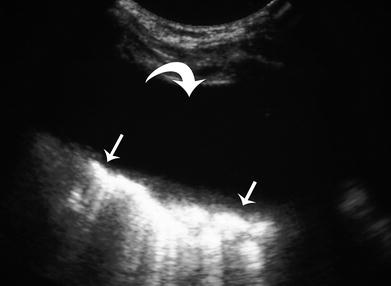

Fig. 4
Ultrasound of simple parapneumonic effusion (curved arrow) in a 3-year-old girl prior to percutaneous catheter drainage over 3 days. Note the lack of fibinous organization or septations. Adjacent consolidated lung (straight arrows) is displaced medially by the effusion
Empyema drainage is usually performed with sonographic rather than CT guidance. Diagnostic chest sonography complements CT in the evaluation of pleural fluid collections, often revealing fibrinous organization, septations, and loculations not revealed by chest CT (Fig. 5). Real-time sonography provides excellent guidance for controlled needle, guidewire, dilator, and catheter placement in the pleural empyema cavity. Sonography is effectively coupled with fluoroscopy for final catheter placement, manipulation, and fluid drainage. A 4-French or 5-French Yueh Centesis needle (Cook Inc, Bloomington, IN) is an excellent pleural access needle and sheath system, which immediately accepts a 0.035 inch guidewire for dilator and catheter placement. Empyemas with fibrinous septations are safely and effectively treated with percutaneous catheter drainage and intracavitary tissue plasminogen activator (tPA) with efficacy of 80–84 % (16–20 % of patients require surgical decortication) (Gates et al. 2004; Gasior et al. 2013). Intracavitary tPA in children is safely and effectively administered as 2 units tPA in 20 ml of saline. The tPA is injected into the catheter, and the catheter is closed for 60 min prior to resumption of drainage. This technique is repeated every 12 h until clearance of residual fibrinous empyema fluid.
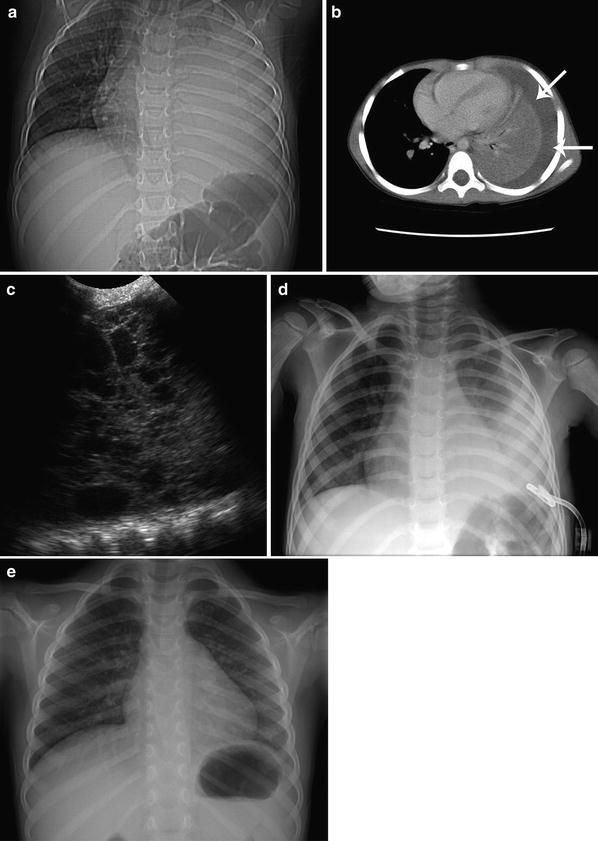

Fig. 5
Left lower lobe pneumonia with empyema in an 8-year-old girl drained with ultrasound guidance. a Supine chest CT localizer radiograph showing dense opacification of the left hemithorax. b Axial chest CT image revealing a left pleural effusion (arrows) without appreciable septations or loculations. c Left hemithorax intercostal ultrasound image obtained during US-guided drainage catheter placement demonstrating the multilocular nature of the empyema with fibrinous organization not revealed by CT. d AP chest radiograph showing a 12F drainage catheter used for tPA fibrinolytic infusion therapy to facilitate empyema drainage. e Upright PA chest radiograph, 1 month following therapy, demonstrating resolution of both the left lower lobe pneumonia and the empyema
3.4 Lung Abscess Drainage
Lung abscesses that have not responded to initial treatment with intravenous antibiotics may be successfully drained with percutaneous catheter drainage techniques. Lung abscesses that abut the pleural surface can be drained safely with little risk of pneumothorax, using techniques and catheters as described for empyema and drainage of other abscesses. As a general rule, abscesses require 8-French or larger catheter systems for effective drainage. Lung abscesses smaller than 3 cm may be effectively drained with a 5F catheter, saline lavage, and a few days of drainage catheter suction (Fig. 6). Lung abscesses that abut the pleural surface are excellent candidates for sonographically guided drainage. In the author’s experience, lung abscesses with persistent bronchial connection following drainage have represented infected congenital bronchopulmonary foregut malformations such as congenital pulmonary airway malformation (CPAM). In these patients, preoperative percutaneous drainage has been used by the author, allowing controlled surgical removal of the CPAM, reducing the risk of intraoperative cyst fluid decompression into the trachobronchial tree of the dependent lung.
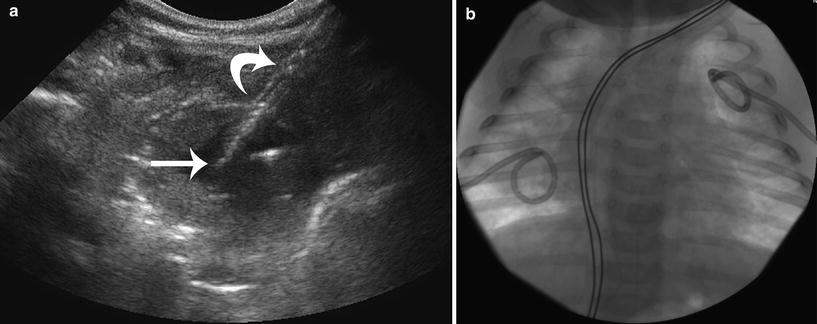

Fig. 6
Ultrasound-guided bilateral lung streptococcal abscess drainages in a 6-month-old boy. a Ultrasound image of the left upper lobe abscess during coaxial placement of a 5F drainage catheter. The 5F pigtail drainage catheter (straight arrow) is seen exiting the coaxial 15G peel-away sheath (curved arrow). b AP chest radiograph showing bilateral 5F drainage catheters that were in place for 3 days prior to cessation of drainage and fever defervescence
3.5 Mediastinal Abscess Drainage
Anterior mediastinal, pericardial, and subpleural abscesses may occur as an extension of complicated deep neck infections. Real-time, high-resolution ultrasound guidance allows millimeter accuracy and control, allowing interventional radiologists the ability to avoid critical structures in the neck and chest during abscess drainage. In the author’s clinical experience, the unossified sternum of infants and children provides for unique and excellent retrosternal visualization and guidance for safe drainage of both pericardial and anterior mediastinal abscesses, as well as vascular malformation treatment access. During anterior mediastinal abscess drainage, sonography is used to guide parasternal needle, guidewire, and drainage catheter placement (Fig. 7). It is important to remember that many mediastinal and subpleural infections that complicate deep neck infections may be limited to a phlegmon, with no drainable fluid, and may respond well to appropriate antibiotic therapy.
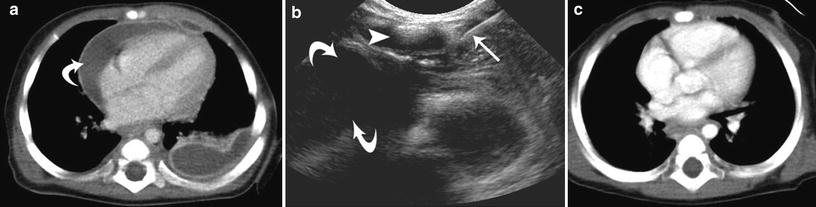

Fig. 7
Ultrasound and fluoroscopic guidance for anterior mediastinal staphylococcal (MRSA) abscess catheter drainage in a 6-month-old girl. a Axial chest CT image demonstrating a pericardial abscess (curved arrow). b Ultrasound image demonstrating a 21G needle (straight arrow) being placed under the unossified sternum (arrowhead) into the pericardial abscess (curved arrows), prior to placement of a 6F drainage catheter over a guidewire. c Axial chest CT image 1 month following abscess drainage, demonstrating complete resolution of the pericardial abscess
3.6 Percutaneous Biopsy
Children presenting with large chest or mediastinal neoplasms (especially anterior mediastinal) may not be candidates for open surgical biopsy, due to the risk of anesthesia associated with airway and/or central vascular compression by the mass (Sola et al. 2013). When consulted by surgeons or oncologists to perform initial diagnostic biopsies in these patients, 14–18 G core biopsy techniques are safely utilized, with either sonographic or CT guidance (Roebuck et al. 2011; Hoffer et al. 1996). Particularly in the chest and mediastinum, freehand sonographic guidance allows real-time control of the procedure, specifically avoiding intrathoracic blood vessels and the lung. Core biopsy procedures are performed using the above described intravenous sedation protocol, supplemented with deep local anesthesia. Deep local anesthesia (Lidocaine 1 %) is most effectively administered under direct sonographic guidance to the level of the tumor or pleural surface. Due to the need for multiple samples of adequate volume for histologic diagnosis, automated core needle biopsy systems are used, placed in a coaxial fashion through a guiding canula. This canula technique allows a single entry site into the tumor, obtaining 3–5 large core samples. Sonography allows precise placement of hemostatic gelatin sponge in the tract as the guiding canula is removed. Large core biopsies have proven sufficient for diagnosis of small cell pediatric tumors (Roebuck et al. 2011; Hoffer et al. 1996).
Fine needle aspirates have not proven adequate for the required battery of diagnostic oncologic tests in most pediatric tumors. However, ultrasound is effective for guiding fine needle aspiration of pneumonia for culture, in cases of indeterminate infectious etiology, as well as guiding biopsy of peripheral and subpleural lung nodules (Fig. 8). Surgical resection (open or thoracoscopic) of small lung nodules is greatly facilitated by immediate preoperative CT or ultrasound-guided needle and guidewire localization (Partrick et al. 2002). A parasternal or anterior intercostal approach is safely used for ultrasound or CT-guided percutaneous anterior mediastinal tumor biopsy (Fig. 9). Middle mediastinal mass biopsy is safely performed by prospective pleural catheter placement and creation of a controlled pneumothorax, allowing the ipsilateral lung to collapse, thus being displaced and allowing an unobstructed path for percutaneous CT-guided biopsy (Lin and Li 2009) (Fig. 10).
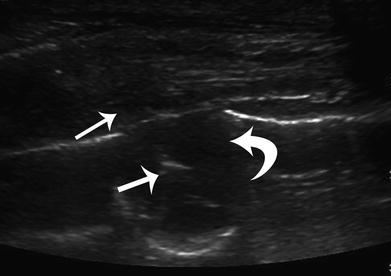
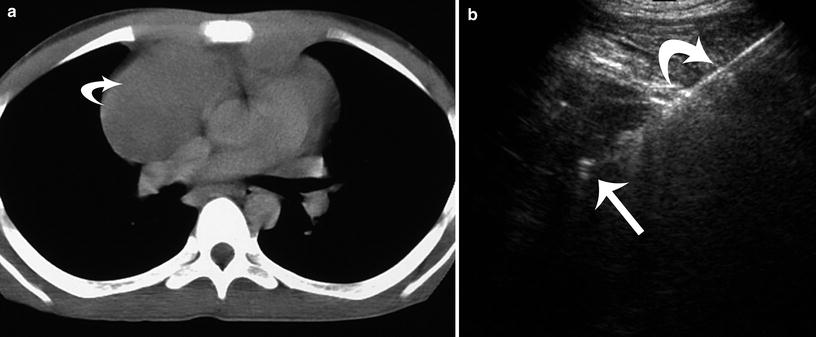
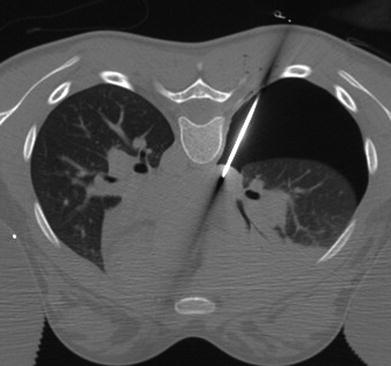

Fig. 8
Ultrasound-guided 16G core biopsy of a subpleural lung nodule in a 16-year-old male. Ultrasound image demonstrating a 16G core biopsy needle (straight arrows) within the subpleural nodule (curved arrow). Histological diagnosis of the nodule was Ewing sarcoma metastasis

Fig. 9
Anterior mediastinal lymphoma with central vascular compression in a 6-year-old boy. a Axial chest CT image demonstrates superior vena caval compression from the anterior mediastinal mass (curved arrow). b Ultrasound image demonstrates the 13G guiding cannula (curved arrow) and the 14 G biopsy needle (straight arrow) during retrieval of five core biopsies for definitive histologic diagnosis

Fig. 10
CT-guided middle mediastinal biopsy with artificial pneumothorax access in a 13-year-old girl. Axial chest CT image with the patient in prone position demonstrates an artificial pneumothorax in the right hemithorax with a 16G core biopsy needle in the mass. Histologic diagnosis was histoplasmosis
3.7 Thermal Ablation of Thoracic Malignancy
Radiofrequency ablation (RFA), microwave ablation, and cryoablation techniques continue to expand providing minimally invasive options for treatment of lung malignancies in children and adults (Shiels and Brown 2005). Unlike adults, children rarely present with primary malignancies of the lung. In the author’s clinical experience, palliative RFA of metastases is currently, and will likely continue to be the most frequent indication for lung tumor thermal ablation in children. In the lung, the author’s experience is focused primarily on the treatment of metastatic osteogenic sarcoma patients who are not operative candidates (Fig. 11). With RFA and microwave ablation, a minimum target temperature of 60 °C over 12 min is required for effective ablation. Ultrasound and CT guidance may be used for lung tumor ablation cases, with CT proving to be most useful when the location of the tumor in the lung precludes an effective sonographic window. The author has safely provided palliative RFA treatment for metastatic lesions as large as 8 cm. The insulating effect of lung limits the extension of necrosis into adjacent tissue. In palliative treatment of metastatic lung lesions, the tissue most resistant to ablation is adjacent to the heart and chest wall, likely due to heat sink effects in these two areas. CT scans demonstrate significant cavitation without pneumothoraces. In the large bilateral tumor ablations, core body temperature elevations are maintained at 38–39 °C or below with the use of a hypothermic blanket system (Medi-Therm II; Gaymar, Orchard Park, NY), with blanket cooling temperatures as low as 20 °C. Following thoracic thermal ablation, it is critical to manage post-procedural pleuritic pain and post-ablation syndrome issues, to include aggressive analgesic and fluid hydration protocols.
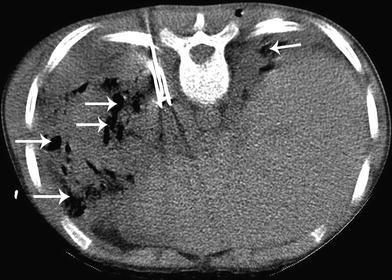

Fig. 11
Radiofrequency ablation (RFA) of bilateral metastatic osteosarcoma in a 16-year-old male. CT-guided RFA procedure with three RFA needles in a left lung mass with air cavitation (arrows) in other bilateral metastatic masses post-ablation
3.8 Chest Arteriography
3.8.1 Aortic Trauma
Aortic injury in the pediatric patient is thankfully rare (Trachiotis et al. 1996; Peclet et al. 1990; Cooper et al. 1994; Eddy et al. 1990; Vignon et al. 1996). Children with traumatic aortic injury are usually pedestrians struck by automobiles and passengers in motor vehicle accidents (Fisher et al. 1997). While mortality for isolated chest trauma in the pediatric population is only 5 %, this increases to 75 % with aortic injury (Peclet et al. 1990; Cooper et al. 1994). Thoracic aortic injury typically involves the arch at the level of the ligamentum arteriosum, although occasionally it may involve the aortic root (Trachiotis et al. 1996; Peclet et al. 1990; Cooper et al. 1994; Eddy et al. 1990; Vignon et al. 1996; Fisher et al. 1997; Pabon-Ramos et al. 2010). Although this injury is uncommon, a high level of suspicion is warranted due to the devastating effects (Eddy et al. 1990). The initial evaluation is typically accomplished with a portable supine radiograph. Signs of mediastinal hematoma, such as a widened mediastinum, indistinctness of the aortic arch, or tracheal deviation may be present, as well as accessory signs such as first rib fracture or apical capping. These findings may be difficult to evaluate in a child who has a disproportionately large thymus and the absence of these findings does not exclude aortic or great vessel injury. Thoracic computed tomography (with CT angiography) and transesophageal echocardiography have been advocated in the adult literature to demonstrate the mediastinal hematoma (Vignon et al. 1996). Improved technology and accuracy of thoracic CT and CT angiography have been associated with reduction in the need to perform diagnostic catheter-based aortography in children (Pabon-Ramos et al. 2010). In cases where there is questionable traumatic aortic injury, catheter-based aortography is indicated. Transfemoral aortography is performed through a vascular sheath. The appropriate size flush catheter (3–5 French) is directed into the supravalvular aortic arch. Small aortas may require the placement of a straight flush catheter. Either digital subtraction or cut film arteriography is performed in two planes, typically left anterior oblique (LAO) and AP. The proximal carotid, brachiocephalic, and subclavian arteries should be included in the imaging field. Injection volume should be 1–1.5 cc/kg over 1–2 s. Rapid filming (3 images/s) should be performed, initially followed by delayed images (1 image/s) to evaluate for areas of focal flow stasis. Care must be taken in the evaluation of the images since ductal diverticula and infundibula of the brachiocephalic arteries can mimic aortic injury (Fisher et al. 1997).
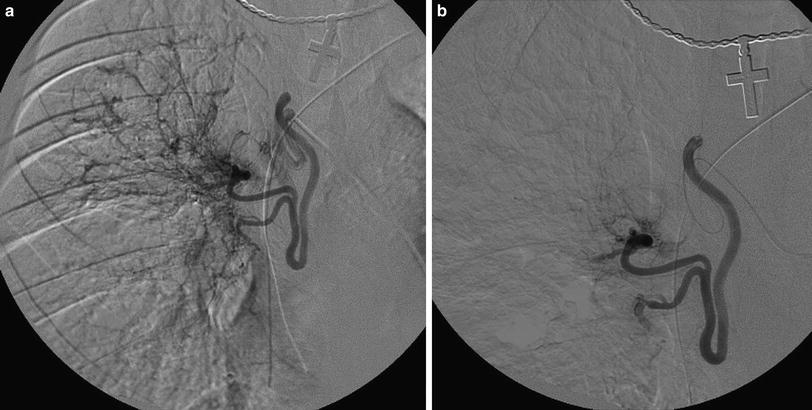
3.8.2 Transcatheter Embolization Treatment of Hemoptysis
Life-threatening hemoptysis in the pediatric population is most commonly a complication of cystic fibrosis (Fellows et al. 1979; Sweezey and Fellows 1990; Cohen et al. 1990; Porter et al. 1983; Cipolli et al. 1995). Traditional therapy had included transfusions, antibiotic therapy, and cessation of percussion and postural drainage (PPD). Percutaneous arteriography with embolization has become an accepted and often preferred method of treatment in these patients. Indications for embolization include severe hemoptysis (> 300 cc in 24 h), recurrent or persistent hemoptysis, or hemoptysis that interferes with the patient’s therapy or lifestyle (Cipolli et al. 1995). Prior to performing this procedure, it is important to explain all complications possible, including the risk of damage to the spinal cord with subsequent neurological compromise (Fellows et al. 1979; Sweezey and Fellows 1990; Cohen et al. 1990; Porter et al. 1983; Cipolli et al. 1995; Barben et al. 2002). While bronchoscopy has been advocated to determine the side of bleeding, the patient often can tell due to either a “funny feeling” or “gurgling” isolated to one side. In the setting of acute life-threatening hemoptysis, bronchoscopy serves little useful purpose and delays appropriate intervention. Hemoptysis is usually from hypertrophied bronchial arteries, usually arising at or near the level of the carina. Multiple collaterals often exist, however, and multiple anastomoses can exist distally in the lung. A vascular sheath is placed in the femoral artery, and 4- to 5-French catheters are advanced into the thoracic aorta near the carina. The bronchial arteries are cannulated and arteriography performed. The images are carefully evaluated to identify the anterior spinal artery. Either the diagnostic catheter or a microcatheter in a coaxial technique is then advanced into the vessel to a point safe for embolization. If a spinal artery is identified, embolization can only be performed if the catheter can be safely advanced distal to its origin. Distal embolization, with either gelatin sponge particles or polyvinyl alcohol particles, is performed to the point of flow stasis (Fig. 12). Supra-distal agents, such as gelatin sponge powder, alcohol, or liquid tissue adhesives should be avoided due to the high risk of bronchial necrosis. Proximal coil embolization, if performed, should only take place after distal embolization. Anastomoses with other collaterals may keep the distal inflammatory bed open, while cutting off access proximally. After embolizing all identified bronchial arteries, we evaluate the subclavian and brachiocephalic arteries to exclude and/or treat other contributing arteries. We perform aortography only if there is difficulty in identifying or cannulating the bronchial arteries, or at completion of embolization to document that all contributaries have been treated. The patient may experience chest pain after the procedure and should be treated with narcotics if necessary. PPD may be restarted after 48 h. Reembolization will be needed in 21–45 % of patients within 1 year (Cipolli et al. 1995; Barben et al. 2002).