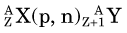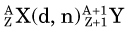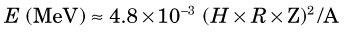chapter 5 Radionuclide and Radiopharmaceutical Production
Most of the naturally occurring radionuclides are very long-lived (e.g., 40K, T1/2 ~ 109 years), represent very heavy elements (e.g., uranium and radium) that are unimportant in metabolic or physiologic processes, or both. Some of the first applications of radioactivity for medical tracer studies in the 1920s and 1930s made use of natural radionuclides; however, because of their generally unfavorable characteristics indicated here, they have found virtually no use in medical diagnosis since that time. The radionuclides used in modern nuclear medicine all are of the manufactured or “artificial” variety. They are made by bombarding nuclei of stable atoms with subnuclear particles (such as neutrons and protons) so as to cause nuclear reactions that convert a stable nucleus into an unstable (radioactive) one. This chapter describes the methods used to produce radionuclides for nuclear medicine as well as some considerations in the labeling of biologically relevant compounds to form radiopharmaceuticals.
a Reactor-Produced Radionuclides
1 Reactor Principles
The “core” of a nuclear reactor contains a quantity of fissionable material, typically natural uranium (235U and 238U) enriched in 235U content. Uranium-235 undergoes spontaneous nuclear fission (T1/2 ~ 7 × 108 years), splitting into two lighter nuclear fragments and emitting two or three fission neutrons in the process (see Chapter 3, Section I). Spontaneous fission of 235U is not a significant source of neutrons or energy in of itself; however, the fission neutrons emitted stimulate additional fission events when they bombard 235U and 238U nuclei. The most important reaction is
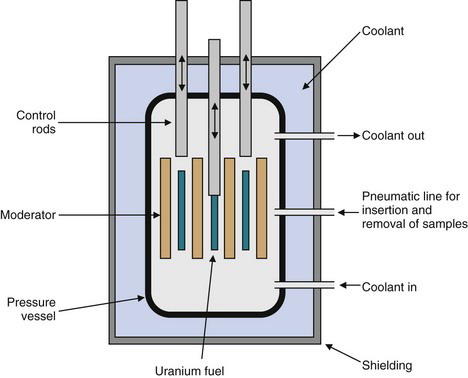
Figure 5-1 is a schematic representation of a nuclear reactor core. “Fuel cells” containing fissionable material (e.g., uranium) are surrounded by a moderator material. The purpose of the moderator is to slow down the rather energetic fission neutrons. Slow neutrons (also called thermal neutrons) are more efficient initiators of additional fission events. Commonly used moderators are “heavy water” [containing deuterium (D2O)] and graphite. Control rods are positioned to either expose or shield the fuel cells from one another. The control rods contain materials that are strong neutron absorbers but that do not themselves undergo nuclear fission (e.g., cadmium or boron). The fuel cells and control rods are positioned carefully so as to establish the critical conditions for a controlled chain reaction. If the control rods were removed (or incorrectly positioned), conditions would exist wherein each fission event would stimulate more than one additional nuclear fission. This could lead to a runaway reaction and to a possible “meltdown” of the reactor core. (This sequence occurs in a very rapid time scale in nuclear explosives. Fortunately, the critical conditions of a nuclear explosion cannot be achieved in a nuclear reactor.) Insertion of additional control rods results in excess absorption of neutrons and terminates the chain reaction. This procedure is used to shut down the reactor.
2 Fission Fragments
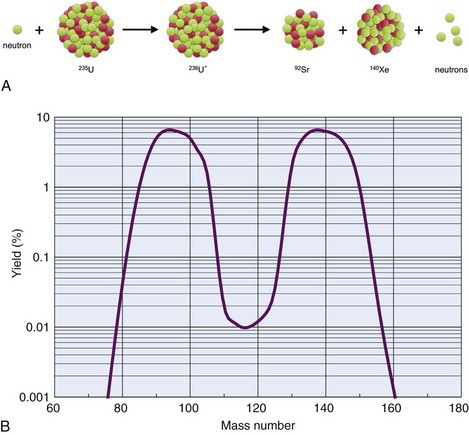
The fission process that takes place in a reactor can lead to useful quantities of medically important radionuclides such as 99Mo, the parent material in the 99mTc generator (see Section C). As described earlier, 236U* promptly decays by splitting into two fragments. A typical fission reaction (Fig. 5-2A) is
More than 100 nuclides representing 20 different elements are found among the fission products of 236U*. The mass distribution of the fission fragments is shown in Figure 5-2B. It can be seen that fission of 236U* generally leads to one fragment with a mass number in the range of 85 to 105 and the other fragment with a mass number in the range of 130 to 150. It also is apparent that fission rarely results in fragments with nearly equal masses.
The half-life of 99Mo is 65.9 hours, which is sufficiently long to allow it to be chemically separated from other fission fragments. Molybdenum-99 plays an important role in nuclear medicine as the parent radionuclide in the 99Mo-99mTc generator (see Section C). Technetium-99m is the most common radionuclide used in clinical nuclear medicine procedures today. Fission has also been used to produce 131I and 133Xe for nuclear medicine studies.
Radionuclides produced by the fission process have the following general characteristics:
3 Neutron Activation
In an (n,γ) reaction a target nucleus, 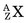 , captures a neutron and is converted into a product nucleus,
, captures a neutron and is converted into a product nucleus, 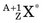 , which is formed in an excited state. The product nucleus immediately undergoes de-excitation to its ground state by emitting a prompt γ ray. The reaction is represented schematically as
, which is formed in an excited state. The product nucleus immediately undergoes de-excitation to its ground state by emitting a prompt γ ray. The reaction is represented schematically as
In these examples, the products (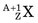 or
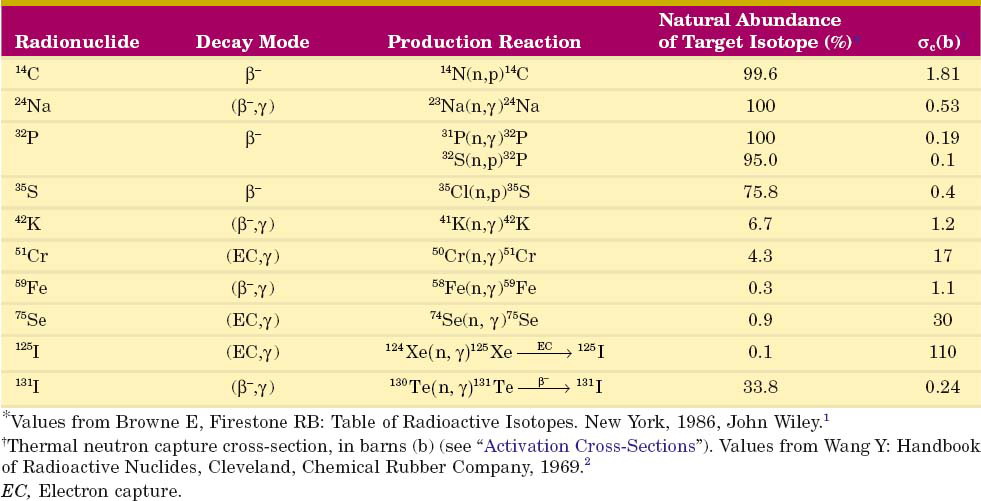
or 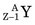 ) usually are radioactive species. The quantity of radioactivity that is produced by neutron activation depends on a number of factors, including the intensity of the neutron flux and the neutron energies. This is discussed in detail in Section D. Production methods for biomedically important radionuclides produced by neutron activation are summarized in Table 5-1.
) usually are radioactive species. The quantity of radioactivity that is produced by neutron activation depends on a number of factors, including the intensity of the neutron flux and the neutron energies. This is discussed in detail in Section D. Production methods for biomedically important radionuclides produced by neutron activation are summarized in Table 5-1.
Radionuclides produced by neutron activation have the following general characteristics:
Lithium-7 is very unstable and promptly disintegrates:
Some of the energetic recoiling tritium nuclei (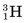 ) bombard stable 16O nuclei, causing the reaction
) bombard stable 16O nuclei, causing the reaction
Useful quantities of 18F can be produced in this way. One problem is removal from the product (by chemical means) of the rather substantial quantity of radioactive tritium that is formed in the reaction. More satisfactory methods for producing 18F involve the use of charged particle accelerators, as discussed in Section B.
b Accelerator-Produced Radionuclides
1 Charged-Particle Accelerators
This reaction can be considered the inverse of the (n,p) reaction that uses neutrons as the bombarding particle and was discussed in Section A.3.
Van de Graaff accelerators, linear accelerators, cyclotrons, and variations of cyclotrons have been used to accelerate charged particles. The cyclotron is the most widely used form of particle accelerator for production of medically important radionuclides.3 Many larger institutions have their own compact biomedical cyclotrons for onsite production of the shorter-lived, positron-emitting radionuclides. The principles and design of cyclotrons dedicated to production of radionuclides for nuclear medicine are described briefly.
2 Cyclotron Principles
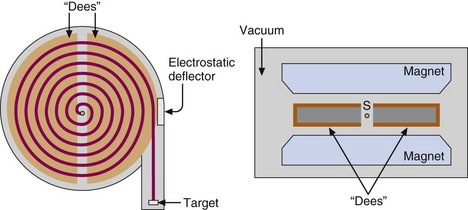
A cyclotron consists of a pair of hollow, semicircular metal electrodes (called dees because of their shape), positioned between the poles of a large electromagnet (Fig. 5-3). The dees are separated from one another by a narrow gap. Near the center of the dees is an ion source, S, (typically an electrical arc device in a gas) that is used to generate the charged particles. All these components are contained in a vacuum tank at ~10–3 Pa(~10–8 atm).
During operation, particles are generated in bursts by the ion source, and a high-frequency alternating current (AC) voltage generated by a high-frequency oscillator (typically 30 kV, 25-30 MHz) is applied across the dees. The particles are injected into the gap and immediately are accelerated toward one of the dees by the electrical field generated by the applied AC voltage. Inside the dee there is no electrical field, but because the particles are in a magnetic field, they follow a curved, circular path around to the opposite side of the dee. The AC voltage frequency is such that the particles arrive at the gap just as the voltage across the dees reaches its maximum value (30 kV) in the opposite direction. The particles are accelerated across the gap, gaining about 30 keV of energy in the process, and then continue on a circular path within the opposite dee.
Each time the particles cross the gap they gain energy, so the orbital radius continuously increases and the particles follow an outwardly spiraling path. The increasing speed of the particles exactly compensates for the increasing distance traveled per half orbit, and they continue to arrive back at the gap exactly in phase with the AC voltage. This condition applies so long as the charge-to-mass ratio of the accelerated particles remains constant. Because of their large relativistic mass increase, even at relatively low energies (~100 keV), it is not practical to accelerate electrons in a cyclotron. Protons can be accelerated to 20-30 MeV, and heavier particles can be accelerated to even higher energies (in proportion to their rest mass), before relativistic mass changes become limiting.*
The energy of particles accelerated in a cyclotron is given by
When the particles reach the maximum orbital radius allowed within the cyclotron dees, they may be directed onto a target placed directly in the orbiting beam path (internal beam irradiation). More commonly, the beam is extracted from the cyclotron and directed onto an external target (external-beam radiation). Typical beam currents at the target are in the range of 50-100 µA. For cyclotrons using positively charged particles (positive ion cyclotron), the beam is electrostatically deflected by a negatively charged plate and directed to the target (Fig. 5-3). Unfortunately electrostatic deflectors are relatively inefficient, as much as 30% of the beam current being lost during extraction. This “lost” beam activates the internal parts of the cyclotron, thus making servicing and maintenance of the cyclotron difficult.
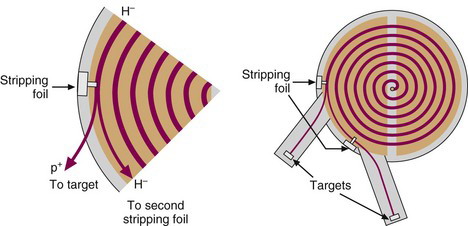
In a negative-ion cyclotron, negatively charged ions (e.g. H–, a proton plus two electrons) are generated and then accelerated in the same manner as the positive ions in a positive-ion cyclotron (but in the opposite direction because of the different polarity). When the negatively charged ions reach the outermost orbit within the dee electrodes, they are passed through a thin (5-25 µm) carbon foil, which strips off the electrons and converts the charge on the particle from negative to positive. The interaction of the magnetic beam with this positive ion bends its direction of motion outward and onto the target (Fig. 5-4). The negative-ion cyclotron has a beam extraction efficiency close to 100% and can therefore be described as a “cold” machine that requires minimal levels of shielding. Furthermore, two beams can be extracted simultaneously by positioning a carbon-stripping foil part way into the path of the beam, such that only a portion of the beam is extracted to a target. The remainder of the beam is allowed to continue to orbit and then is extracted with a second stripping foil onto a different target (Fig. 5-4). This allows two different radionuclides to be prepared simultaneously. One disadvantage of negative-ion cyclotrons is the requirement for a much higher vacuum (typically 10–5 Pa compared with 10–3 Pa for positive ion machines) because of the unstable nature of the H– ion, the most commonly used particle in negative ion cyclotrons.
3 Cyclotron-Produced Radionuclides
Cyclotrons are used to produce a variety of radionuclides for nuclear medicine, some of which are listed in Table 5-2. General characteristics of cyclotron-produced radionuclides include the following:
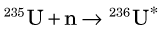
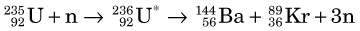
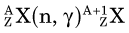
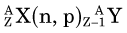
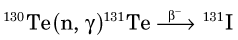
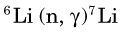
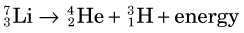
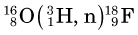
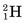 nuclei), and α particles (
nuclei), and α particles (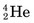 nuclei), to very high energies. When directed onto a target material, these particles may cause nuclear reactions that result in the formation of radionuclides in a manner similar to neutron activation in a reactor. A major difference is that the particles must have very high energies, typically 10-20 MeV, to penetrate the repulsive coulomb forces surrounding the nucleus.
nuclei), to very high energies. When directed onto a target material, these particles may cause nuclear reactions that result in the formation of radionuclides in a manner similar to neutron activation in a reactor. A major difference is that the particles must have very high energies, typically 10-20 MeV, to penetrate the repulsive coulomb forces surrounding the nucleus.