Fig. 1
Well-differentiated neuroendocrine tumor (NET) of pancreatic tail, s.p. extended pancreatectomy and partial omentectomy, initial tumor stage (UICC) pT2 (Ø 3.2 cm) pN1 (6/12) cM1 R0, LX VX, G2, proliferation rate (Ki-67) 10 % and immunohistochemical expression of chromogranin A, synaptophysin, and somatostatin receptors (SSTRs), underwent peptide receptor radionuclide therapy (PRRT). Strongly SSTR-positive liver metastasis in S1 (SUV 26.1) on 68Ga-DOTATOC PET/CT before therapy (a coronal fused; c transverse fused), hypodense on CT (b transverse view), seen on T-2 weighted MRI (d transverse view) and importantly, ametabolic on 18F-FDG PET/CT with complete mismatch (e transverse fused image)
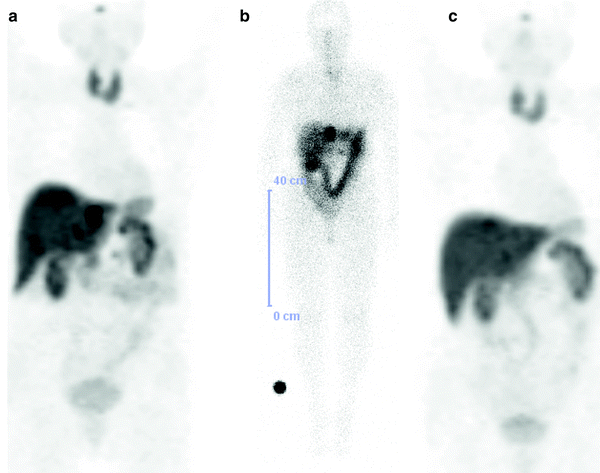
Fig. 2
(Fig. 1 continued) The 68Ga-DOTATOC PET MIP image before PRRT (a) shows in addition to the receptor-positive lesion in S1, discrete tracer uptake in S6. The 177Lu-DOTATOC whole-body planar scan 44 h after therapy also demonstrates an intense uptake in the liver metastasis described above. There was an excellent response after 1 PRRT cycle, as seen on the 68Ga-DOTATOC PET image after therapy (c)
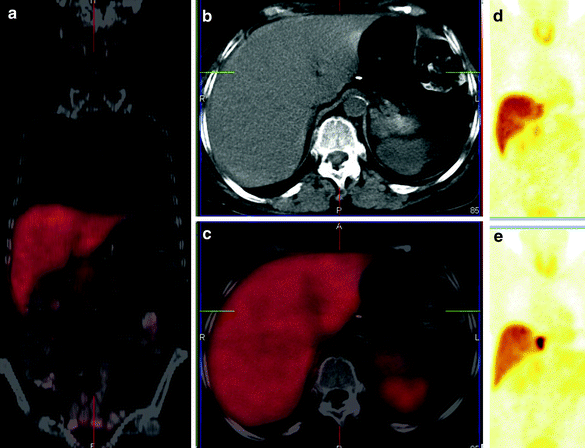
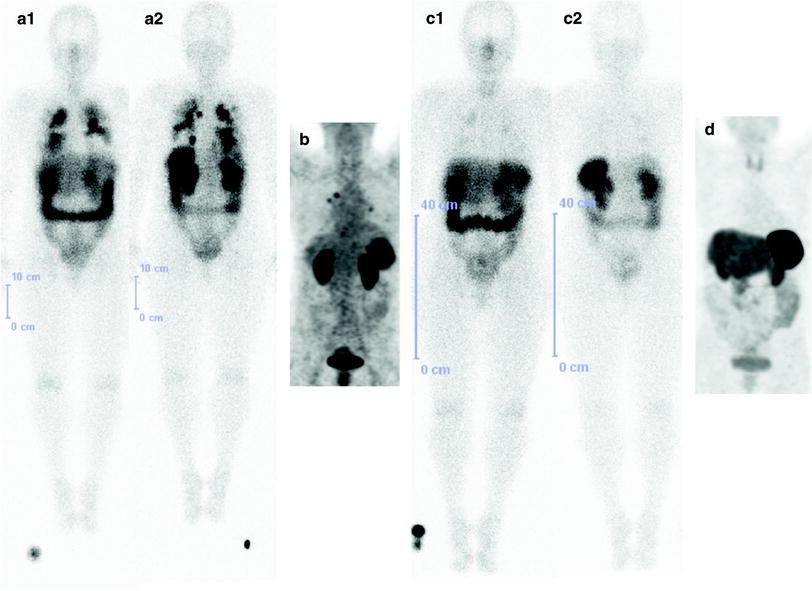
Fig. 4
A 68-year-old female with well-diffferentiated, nonfunctional NET (DD primary in the liver) with widespread metastasis in the right hepatic lobe (tumor size 12 × 10 × 10.5 cm) and, s.p. right hemihepatectomy, partial omentectomy, and cold octreotide therapy presented with bilateral pulmonary metastases. She underwent two cycles of PRRT with a cumulative administered activity of 12.3 GBq 177Lu-DOTATATE. The post-therapy whole-body scan after the first PRRT cycle (a1 anterior view; a2 posterior view) showed extremely high uptake in the intrapulmonary metastases bilaterally. A response to PRRT was already noted after the first PRRT cycle (b 68Ga-DOTATOC PET MIP) and a striking difference was noted in the post-therapy whole-body scan after second PRRT cycle (c1 anterior view; c2 posterior view). A complete remission was noted after the second PRRT cycle (as shown in the 68Ga-SSTR PET MIP image, d)
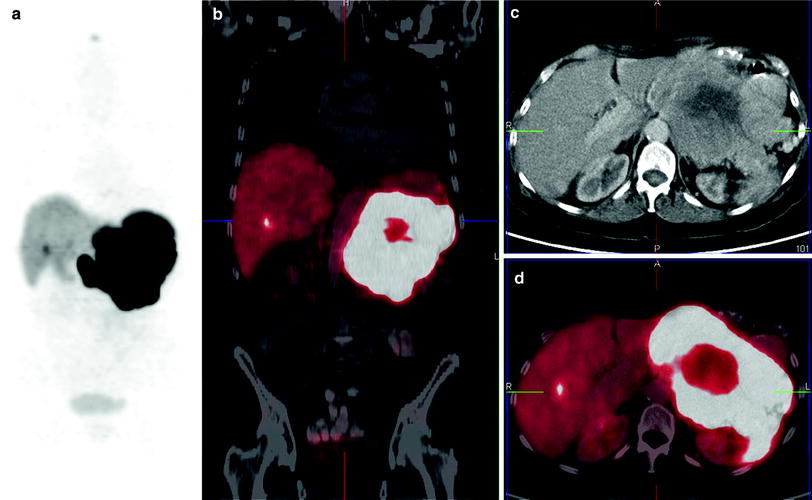
Fig. 5
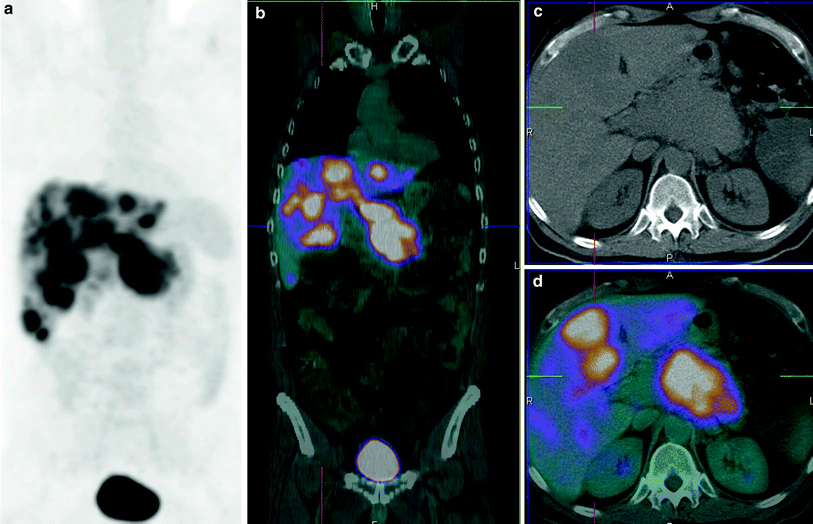
Well-differentiated, nonfunctional pancreatic NET with infiltration of the surrounding structures and displacement of the abdominal organs, tumor-induced portal vein thrombosis and metastatic osseous and liver disease, initial tumor stage IV (UICC) and proliferation (Ki-67) 4 % (G2). Three cycles of PRRT were performed, first two with 90Y-DOTATOC (due to the large size of tumor), a cumulative administered activity of 10 GBq. The second cycle was intraarterial, targeting the primary tumor specifically ensuring high dosing. This figure demonstrates the pretherapy 68Ga-DOTATOC PET/CT is shown here (a MIP; b coronal fused, c transverse CT; d transverse fused)
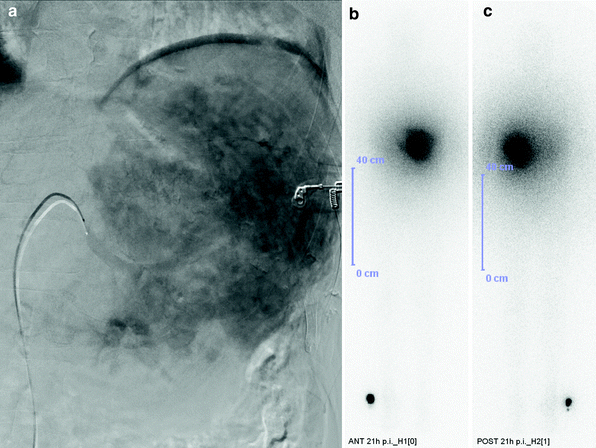
Fig. 6
(Fig. 5 continued) a shows the targeting of the primary tumor demonstrated by the angiography followed by the 90Y-DOTATOC Bremsstrahlung scans (b anterior view; c posterior view)
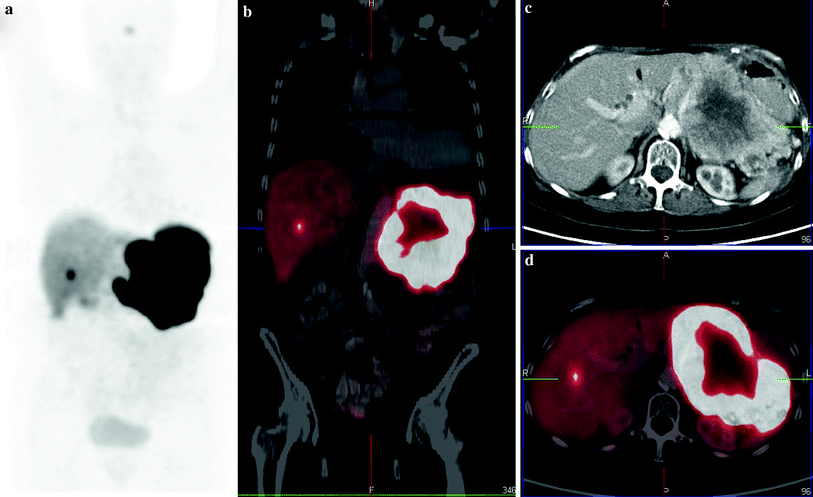
Fig. 7
(Figs. 5 and 6 continued) As compared to the pretherapy 68Ga-DOTATOC PET/CT (shown in Fig. 5), there was a significant decrease of uptake in the primary tumor by 27 % as revealed by the post-therapy 68Ga-DOTATOC PET/CT (a MIP; b coronal fused, c transverse CT; d transverse fused), i.e., partial response according to molecular imaging criteria
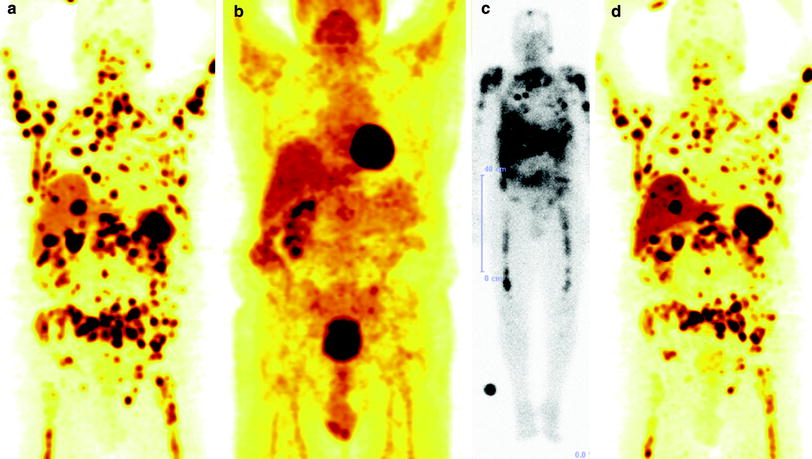
Fig. 8
A 67 year-old male with functional NET of the rectum (Ghrelinoma, initial tumor stage IV) first diagnosed 8 years back, presented to our department with disseminated osseous metastasis and extensive organ (liver/kidney), soft tissue, and lymph node metastases. He had previously undergone sigmoidostomy, percutaneous radiotherapy (4 × 6 Gy = 24 Gy) to thoracic and lumbar vertebrae (T4-10 and L2-4), palliative chemotherapy with Streptozotocin, 5-FU and doxorubicin, and four cycles of PRRT at another center (total administered activity 32 GBq 177Lu). This was followed in course by Octreotide therapy (Sandostatin LAR) and local external radiotherapy (15 × 3 Gy = 45 Gy) for local recurrence in the presacral region and in the rectum with evidence of perforation. The fifth cycle of PRRT was then administered at the same center with 8 GBq Lu-177 (cumulatively 40 GBq, renal dose 40 Gy), but the osseous metastases progressed. He was diagnosed here to have a functional single kidney (a hydronephrotic and atrophied left kidney) after a 99mTc-MAG3 scan performed at our center. He underwent two further cycles of PRRT at our center with 5 GBq each [cumulative administered activity 50 GBq (1,351 mCi) of 177Lu over 7 cycles], with partial remission of the disease according to molecular response criteria. (a 68Ga-DOTATOC PET MIP image before the sixth PRRT; b 18F-FDG PET MIP image before the sixth PRRT showing a complete mismatch, i.e., absence of glucose hypermetabolism in the disseminated metastases; c 177Lu-DOTATATE post-therapy whole-body scan after the seventh PRRT cycle; d 68Ga-DOTATOC PET MIP image after the seventh PRRT)
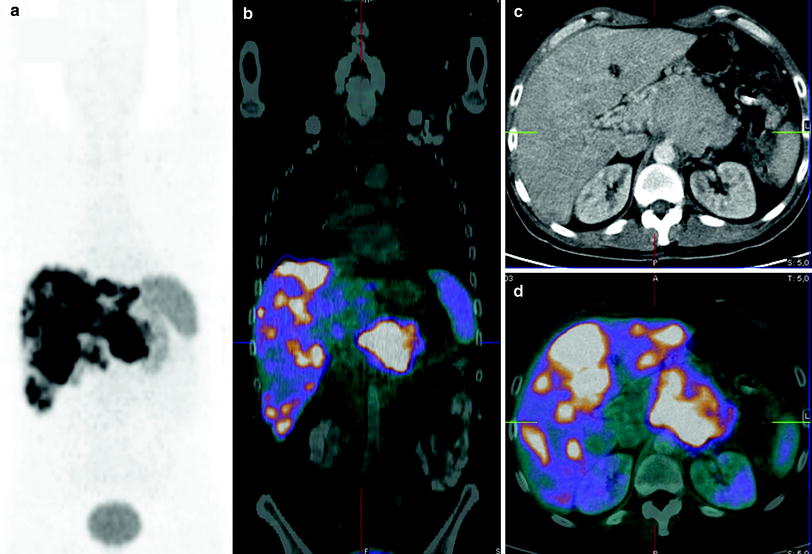
Fig. 9
Well-differentiated functioning NET (VIPoma with Verner Morrison syndrome) of the pancreatic body with progressive multiple liver metastases, expression of synaptophysin and chromogranin and proliferation rate (Ki-67) of 10 %. PRRT was performed in three cycles with total administered radioactivity 21.8 GBq 90Y/177Lu, the second and third cycles being tandem (combination of 90Y and 177Lu). At the end of third cycle, according to molecular response criteria there was stable disease/minor response concerning the primary tumor, and partial remission with decreasing SSTR expression of the known liver metastases. No new or extrahepatic receptor-positive lesions were noted. CT scan showed stable disease according to RECIST criteria. Interestingly, 18F-FDG PET/CT showed a remarkable improvement with decreased hypermetabolism in the liver metastases as well as the primary tumor (Figs. 9, 10, 11, 12, 13). This figure shows pretherapy 68Ga-DOTATOC PET/CT
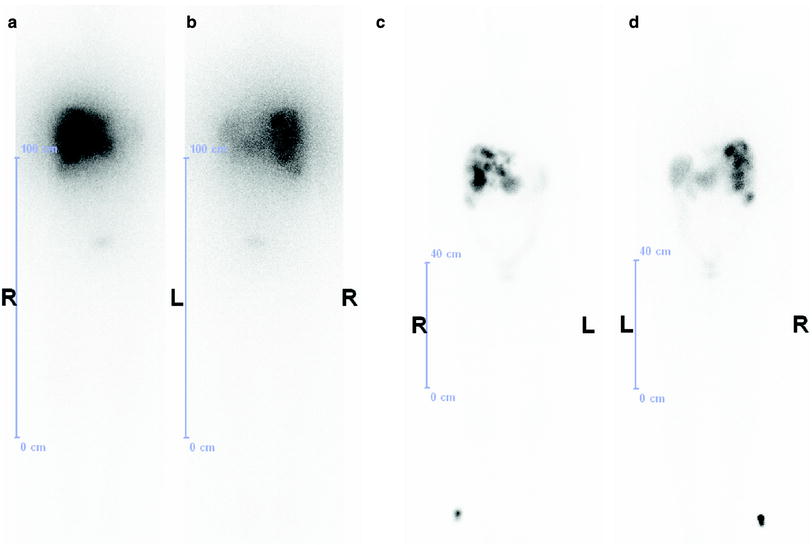
Fig. 11
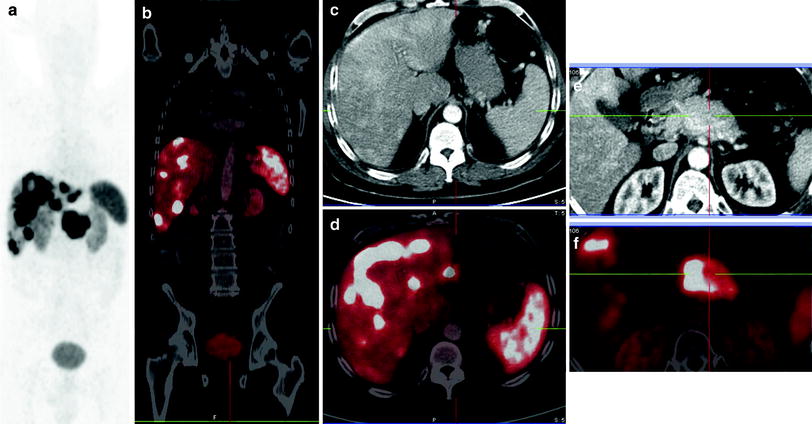
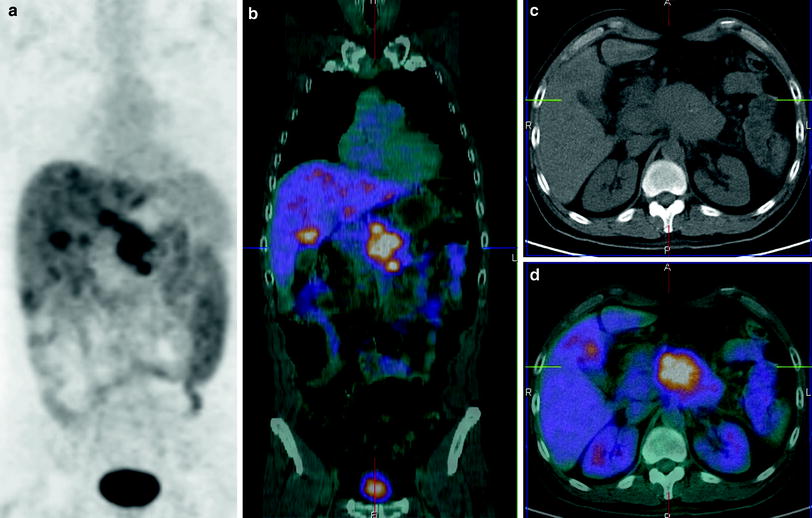
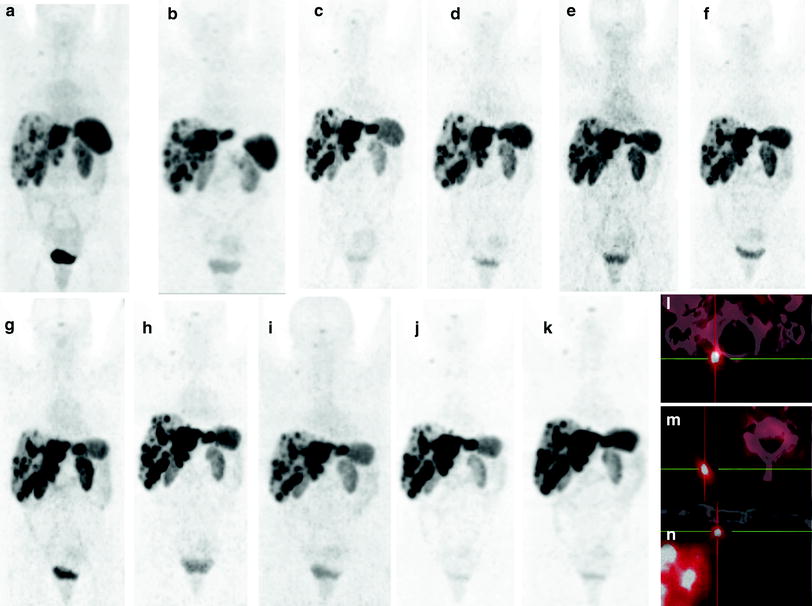
Fig. 14
Long-term course of an initially progressive NET: “Do not treat if stable”. A 42-year-old female patient diagnosed with NET G2 of the ileum with infiltration of the surrounding fat, appendix, lymphangiosis and hemangiosis carcinomatosa, regional lymph node and hepatic metastases, s.p. surgery underwent first cycle of PRRT 1 year after the diagnosis of progressive lymph node and hepatic metastases. She underwent six cycles of PRRT over the next 2 years using 17.6 GBq 90Y-DOTATATE. Not only was the disease stable over the following 7 years (9 years since the first PRRT cycle) as demonstrated by serial 68Ga-SSTR PET/CT but also the renal function was normal as demonstrated by serial monitoring. a–k, serial MIP images, a first follow-up 4 months after the sixth PRRT, and k 9 years after the 6th PRRT with respect to the SSTR-positive multiple hepatic metastases, notably rare soft-tissue metastases right lateral to the foramen magnum (l), in the right trapezius muscle (m) and lymph node metastases, for e.g., internal mammary, n (l, m, n, fused transverse PET/CT images)
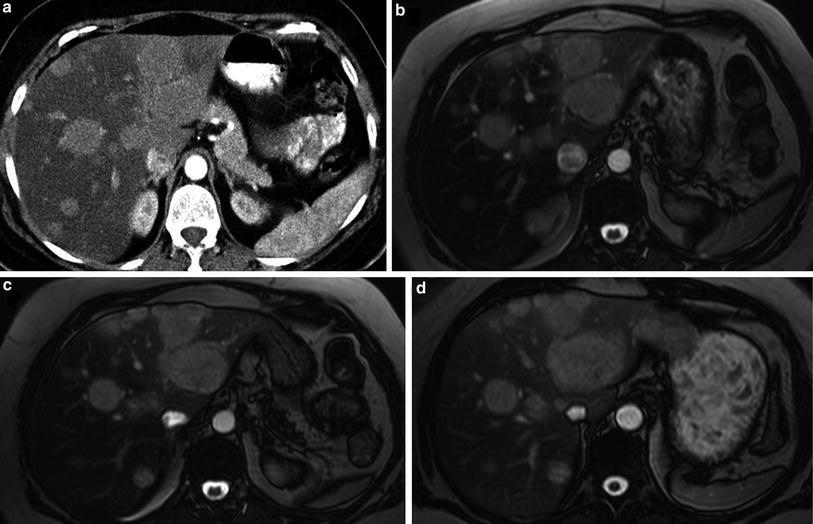
Fig. 15
Long-term course of an initially progressive NET: “Do not treat if stable”. A 42-year-old female patient diagnosed with NET G2 of the ileum with infiltration of the surrounding fat, appendix, lymphangiosis and hemangiosis carcinomatosa, regional lymph node and hepatic metastases, s.p. surgery underwent first cycle of PRRT 1 year after the diagnosis for progressive lymph node and hepatic metastases. She underwent six cycles of PRRT over the next 2 years using 17.6 GBq 90Y-DOTATATE. This figure shows stable disease with respect to the liver metastases, also according to RECIST criteria, as demonstrated by serial CT/MRI examinations. (a CT during first follow-up after sixth PRRT and b, c, d, serial MRI scans, d being the last follow-up scan 9 years after the first PRRT)
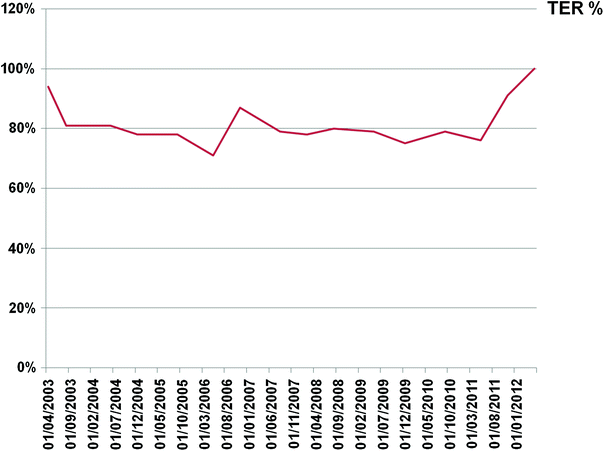
Fig. 16
Long-term follow-up of renal function after PRRT: A 45-year-old patient with extensive metastatic glucagonoma involving the pancreatic head and with documented erythema migrans necrolyticum, s.p. Whipple’s procedure, chemotherapy (six cycles of dacarbazine), hepatic alcohol injection (a total of 11 courses), hemihepatectomy, following empyema (decortication of the right lung), and liver abscess, hereafter transarterial chemoembolization (TACE) of liver metastases, resection of liver segment 1/2, and resection of mesenteric lymph node metastases. The patient was treated with nine PRRT cycles, without any radiation-induced nephropathy, i.e., without any tubular or glomerular dysfunction. The tubular extraction rate. TER, was normal after 9 years
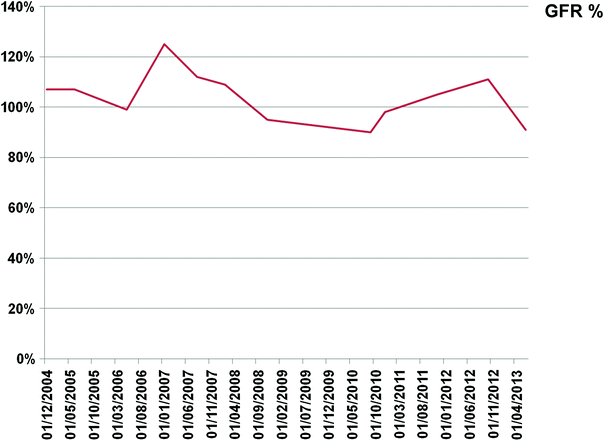
Fig. 17
This figure shows the course of glomerular filtration rate, GFR, which remained stable over 9 years, in the same patient as described under Fig. 16
2 Radionuclides
High doses of the radiopeptide [111In-DTPA0]-octreotide were first applied for the radionuclide therapy in NETs (Krenning et al. 1994). However, due to the short range (nanometers to micrometers) of the emission and therefore the short tissue penetration of the emitted Auger electrons, objective responses were rare. Furthermore, leukemia or myelodysplastic syndrome has been reported in three patients from the group receiving the highest cumulative dose (Valkema et al. 2002). 111In-pentetreotide is still used by some departments in the USA, mainly due to lack of accessibility to ß-emitting radiotracers. In fact, a recent study reported safety and survival benefit in more than 100 patients treated with 111In-pentetreotide (Delpassand et al. 2012).
The most frequently used radionuclides in PRRT are 90Y and 77Lu. 90Y is produced as a carrier-free radionuclide from the parent radioisotope 90Sr. The radioisotope 177Lu is produced by nuclear reactors either via the direct or the indirect pathway; the direct pathway involves the n-gamma irradiation of an enriched 176Lu target. The indirect pathway involves the irradiation of Ytterbium-176 (176Yb) to produce the short-lived 177Yb (half life, t1/2 1.9 h) that decays to 177Lu. The latter method avoids the production of the long-lived 177mLu (t1/2 60 days).
These radionuclides have different physical characteristics, for e.g., beta particles are emitted at different energies, resulting in various tissue penetration ranges. A pronounced “cross-fire effect” is found when using 90Y, since the beta particles are emitted with a relatively high energy (maximum energy, 2.27 MeV), resulting in a tissue penetration range of up to 12 mm. This may be preferable for relatively uniform irradiation of larger tumors, which may show heterogeneous uptake. Due to its pure β-emission, dosimetric analysis has to be performed using a surrogate like 111In. The use of 86Y and 90Y PET or bremsstrahlung imaging has also been reported for dosimetry (Delpassand et al. 2012). 177Lu on the other hand, emits intermediate-energy beta particles (maximum energy 0.5 MeV), resulting in a tissue penetration range of up to 2 mm, which may be preferable for smaller tumors. In addition, 177Lu has two gamma peaks at 113 and 208 keV with 10 and 6 % abundance respectively, which makes it suitable for imaging with a gamma camera for post-therapeutic dosimetry with the same radiopharmaceutical. Another difference between these radionuclides is their physical half-life. Although the influence of the physical half-life is not yet fully understood, it is very likely that it influences the therapeutic as well as the secondary effects. For 90Y, it is 2.7 days, whereas the physical half-life of 177Lu is more than double (6.7 days). These differences in characteristics in turn influence the decision on the choice of radionuclide to be used for PRRT.
3 Chelators and Peptides
Chelators are essential for the radiolabeling of SST analogs. The macrocyclic chelating agent DOTA (1, 4, 7, 10-tetraazacyclododecane-N, N′, N″, N‴-tetraacetic acid) forms the most widely used complexes for diagnosis and therapy of NETs, namely [DOTA0,Tyr3] octreotide (DOTATOC) and [DOTA0,Tyr3] octreotate (DOTATATE) for complexing trivalent radiometals, i.e., 68Ga (PET) and 177Lu/90Y (PRRT) for the theranostics of NETs. The radiochemical properties of diethylenetriaminepentaacetic acid (DTPA) are favorable for chelation with 111In; however, 90Y-DTPA has poor in vivo stability (Heppeler et al. 2000). 90Y-DOTA, on the other hand, is a highly stable complex with enhanced thermodynamic and kinetic stability relative to an open-chain analog (Moi and Meares 1988; Deshpande et al. 1990). Due to the high affinity for SSTR 2, DOTATOC has favorable potential for therapeutic use (Otte et al. 1997; Paganelli et al. 1999). Moreover, it has high hydrophilicity, stability, and is suitable for labeling with 90Y and 111In (de Jong et al. 1998).
DOTATATE has a nine-fold higher affinity for the SSTR 2 compared to DOTATOC (Reubi et al. 2000). DOTANOC ([DOTA 0–1-Nal 3]octreotide) exhibits the highest affinity to SSTR 3 and 5, and a high affinity to the SSTR 2 (Wild et al. 2003). Wehrmann et al. compared the kinetics and dosimetry of 177Lu-DOTATATE and 177Lu-DOTANOC and concluded that whole-body and normal tissue uptake was higher for DOTANOC, resulting in a higher mean absorbed dose to the whole body. Hence though the kidney and tumor doses were comparable for the two radioligands, 177Lu-DOTATATE has characteristics more favorable for PRRT than 177Lu DOTANOC, because a lower whole-body dose implies a lower potential bone marrow toxicity (Wehrmann et al. 2007). Esser et al., in their study comparing 177Lu-DOTATATE and 177Lu-DOTATOC in seven patients, had found a longer residence time in the kidneys with DOTATATE, but the ratio of residence times of DOTATATE to DOTATOC in the tumor (2.1) was higher than that for the kidneys; hence, DOTATATE was found to deliver a higher dose to the tumor (Esser et al. 2006). However, another recent study comparing the two peptides in 22 patients concluded that the tumor-to-kidney ratio was comparable for both peptides (Kulkarni et al. 2013). Though uptake, residence time and mean absorbed renal dose per unit administered activity showed significantly higher values for DOTATATE in 19 (86 %) patients, both the peptides were found to be safe in terms of potential for renal toxicity. On the other hand, Forrer et al. demonstrated no difference in the tumor uptake of 111In-DOTATATE and 111In-DOTATOC, whereas a higher tumor-to-kidney absorbed dose ratio for 111In-DOTATOC (Forrer et al. 2004).
4 Patient Selection for PRRT
Curative treatment of NETs usually requires the possibility of complete surgical resection of the primary tumor and perhaps regional lymph node metastases. Surgery and cold somatostatin analogs (i.m. or s.c. octreotide or lanreotide) are most commonly used as the first line of treatment; chemotherapy is used as the last option or in high-grade tumors. Recent years have seen the development of PRRT as a highly effective treatment option for inoperable or metastasized progressive well-differentiated NETs. The WHO grade of the tumor determined by the Ki-67 tumor proliferation index is essential for patient selection (Ezziddin et al. 2011). G1 and G2 tumors are known to respond well to PRRT. Also, high somatostatin receptor expression of tumors must be established before therapy, in order to ensure therapeutic efficacy. This requires semiquantitative interpretation (Krennings’ score) with somatostatin receptor scintigraphy using 111In-Octreotide, or with SSTR PET/CT using 68Ga-DOTA-peptides. Although dosimetry is the best way to “individualize” PRRT, semiquantitative evaluation of standardized uptake values (SUVs) of the tumors on SSTR PET/CT, is an excellent measure of the degree of SSTR expression (Kaemmerer et al. 2011). A recent study established a correlation between the SUV on pre-PRRT SSTR PET and tumor dose during PRRT (Ezziddin et al. 2012). A trend in terms of the tumor dose–response relationship during PRRT was demonstrated by another preliminary study (Kulkarni et al. 2011). Hence, pretherapeutic assessment of the tumor SUV could potentially help to predict the response to PRRT based on the knowledge of this dose–response relationship and lead to better selection of the patients for PRRT. However, further studies are required to examine this early evidence.
Severi et al. further determined the prognostic value of 18F-FDG PET in 52 patients with progressive advanced well-differentiated grade 1/2 NETs treated with 177Lu-DOTATATE (Lu-PRRT). They found that none of PET-negative patients had progressed at the first follow-up examination after 177Lu-PRRT, whereas grade 2 NET and 18F-FDG PET positivity (arbitrary SUV cutoff >2.5) were frequently associated with more aggressive disease, suggesting perhaps the requirement of a more intensive treatment regimen for the latter group (Severi et al. 2013).
In a recent study by Campana et al., 69 NET patients (37 men and 32 women; 45 pancreatic and 24 gastrointestinal; 22 G1 and 41 G2) were treated with 90Y or 177Lu. Partial response (PR) was noted in 27.5 % patients, while 50.7 % had stable disease and 23.2 % had progressive disease. Low tumor burden and a low proliferation index represent independent prognostic factors for long PFS while stage IV, NET G2, and previous TACE were found to be significant factors for tumor progression at multivariate analysis (Campana et al. 2013).
5 Dosimetry
The challenge for internal radiation therapy is to deliver the highest possible dose to the tumor, while sparing normal organs from damage. In addition, the interindividual differences in dose delivery should be considered, particularly on the account of varying metabolism or receptor density in organs and tumor lesions. This makes individual patient dosimetry absolutely necessary in PRRT. The advantage of 177Lu is the concomitant emission of low-energy γ-rays at 208 and 113 keV with the therapeutic β-radiation, which allows not only assessment of biodistribution with post-therapy scans, but also enables individualized dosimetry. It is imperative to calculate the absorbed radiation doses to dose-limiting organs such as the kidneys and bone marrow to better influence the decision for administering multiple cycles of PRRT, taking into account the cumulative administered activity. Taking into consideration the highly variable SSTR densities on tumor cells and other factors like tumor size and viability, individualized dosimetry helps in patient selection and therapy planning.
The data required as input for dosimetry are blood and urine samples, and whole-body scans at adequate time intervals up to at least 3 days post PRRT. Regions of interest (ROI) on planar images are useful to obtain the time–activity curves. However a much better organ-specific three-dimensional activity distribution is provided by SPECT or SPECT/CT images, although the acquisition is time consuming. The dosimetry estimation is based on the MIRD scheme (Siegel et al. 1999). Using dedicated software like OLINDA/EXM, mean absorbed dose estimates can be derived based on the calculated residence time of radiopeptide used (Stabin et al. 2005).
The lack of gamma emission by 90Y is a drawback so as to enable direct dosimetric analysis. Quantification using bremsstrahlung images is difficult and requires complex corrections. An excellent alternative is use of the positron emitter 86Y (Walrand et al. 2011). The advantage of using 86Y-DOTATOC is the accurate mimicking of the in vitro pharmacokinetics and a much superior resolution and accurate quantification by PET. However, the physical half-life (14.7 h) and low positron abundance of 86Y does not permit delayed imaging, and the high production cost and low availability considerably limits its routine utilization. Due to the half-life of 111In and the pharmacokinetics, 111In-DOTATOC is used in clinical practice as a surrogate for 90Y-DOTATOC. 111In-pentetreotide scintigraphy, however, is not suitable for accurate dosimetric calculation due to its different kinetic behavior and receptor affinity profile. The short physical half-life of 68Ga also makes pretherapeutic dosimetry unsuitable. The use of long-lived positron emitters, e.g., 44Sc or 64Cu for receptor PET/CT could enable pretherapeutic organ and tumor dosimetry by comparison of these results with those obtained from 68Ga-SSTR PET/CT results. The appropriate peptide and radionuclide for individualized therapy could then be selected by pretherapeutic measurement of organ and tumor doses.
6 Kidney Protection
Due to the small size, radiopeptides are filtered through glomerular capillaries in the kidneys and subsequently reabsorbed and retained in the proximal tubular cells. Kidneys are the dose-limiting organs for PRRT, due to their marked radiosensitivity (Bodei et al. 2004; Valkema et al. 2005). While the cross-fire effect is beneficial to overcome the inhomogeneity of receptor expression in cancer cells, allowing irradiation of tumor cells which are not directly targeted by the radiopharmaceutical, the long range of the 90Y beta particles appears to further increase the potential for kidney toxicity. Cases of severe, end-stage renal disease have been reported with high activities of 90Y at early stage of introduction of PRRT (Cybulla et al. 2001). However, adequate renal protection can minimize the risk of renal damage. Positively charged molecules such as l-lysine and/or l-arginine competitively inhibit the proximal tubular reabsorption of the radiopeptide and hence are co-administered with PRRT, having shown to reduce the renal dose by 9–53 % (Bernard et al. 1997; Rolleman et al. 2003). The dose reduces further up to 39 % when the infusion is prolonged over 10 h and up to 65 % by prolonging it over 2 days after radiopeptide administration, thereby covering almost the entire elimination phase through the kidneys (Bodei et al. 2004). The patients should also be well hydrated. Gelofusine, in addition to the amino acids, also inhibits the reabsorption of these peptides and has been shown to reduce the renal uptake significantly (Rolleman et al. 2008). The role of radioprotective drugs has also been investigated in preclinical studies. Amifostine has been demonstrated to alleviate the radiation-induced renal damage during therapy with 177Lu-DOTATATE (Rolleman et al. 2007). The same group also recently established significant reduction in the mean absorbed renal dose with 111In-DOTATATE when amifostine was co-administered, which could have mitigated the renal damage (Melis et al. 2012).
Despite renal protection, there is a risk of renal toxicity (usually mild) months after irradiation. A decline in creatinine clearance with time has been demonstrated, more pronounced with 90Y-labeled peptides (median 7.3 % per year), but also for 177Lu (median 3.8 % per year) (Valkema et al. 2005). Cumulative and per-cycle renal absorbed dose, age, hypertension, and diabetes were found to be contributing factors to the decline of renal function after PRRT. The maximum tolerated renal dose according to the studies with external beam therapy was considered to be 23–27 Gy (Cassady 1995). However, in internal radiation therapy, which involves different dose rates, biologically equivalent dose (BED) was found to more accurately predict the renal toxicity (Dale 2004). Barone et al. showed that the individual renal volume, dose rate, and fractionation, which are taken into account by BED, were able to predict renal function impairment (Barone et al. 2005). A much lower threshold of (BED) to the kidney, i.e., 28 Gy in patients with risk factors (mainly hypertension and diabetes), than in patients without risk factors (40 Gy) predicts renal toxicity after PRRT (Bodei et al. 2008).
A large retrospective study of 1,109 patients treated with 90Y-DOTATOC by the Basel group found a 9 % prevalence of permanent renal toxicity (Imhof et al. 2011). This could have been due to the rather high activities of 90Y administered per cycle (3.7 GBq/sq. m body surface area). Fractionation of PRRT, as in the case of external beam radiotherapy may be safer since internal radiation therapy leads to a continuous radiation delivery at a low and decreasing dose rate over time, which may allow the normal tissues like kidney to repair the radiation damage (Dale and Carabe-Fernandez 2005). With a long-term low-dose protocol, administering multiple (up to 8) cycles per patient and adequate nephroprotection (co-administering gelofusine additionally in case of 90Y), no significant renal toxicity, i.e., end-stage renal insufficiency has been reported by the Bad Berka group in more than 1,000 patients treated with 177Lu and/or 90Y (>1,500 treatment cycles using 90Y) (Baum and Kulkarni 2012). In 2009, two patients on hemodialysis due to end-stage renal insufficiency and with progressive NET after exhausting all other treatment options, were treated with fractionated low-dose PRRT using 177Lu at our center (first ever worldwide experience with such a condition to the best of the authors’ knowledge). No significant hematotoxicity was observed in either of these patients on dialyses and both showed a good clinical and objective therapy response. A recent study of a cohort of 51 patients treated with up to 29 GBq of 177Lu also revealed no major acute or delayed renal toxicity, demonstrating the safe use of 177Lu (Bodei et al. 2011). PRRT should only be performed at specialized centers as NET patients need highly individualized interdisciplinary treatment and long-term care (Hörsch et al. 2011).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree