Fig. 3.1
Target pathways for antiangiogenic therapy in NETs. Image depicts the cellular and molecular components that drive angiogenesis in NETs (tumor cells, endothelial cells, pericytes, and extracellular matrix). Furthermore, in order to block the main pro-angiogenic pathways (VEGF/VEGFR and PDGF/PDGFR), different drugs such as endogenous inhibitors (endostatin), antibodies (bevacizumab) or small molecule inhibitors (sunitinib, sorafenib, vatalanib, and pazopanib) that can target vascular or perivascular cells have been developed
The VEGF/VEGFR Axis
The key mediator of angiogenesis is the VEGF, and VEGF signaling inhibition has been shown to result in significant tumor growth delay in a wide range of animal models [14]. The inhibition of VEGF signaling not only arrests endothelial cells (ECs) proliferation and prevents vessel growth, but also induces regression of existing vessels by increasing EC death. VEGF inhibitors also suppress the mobilization of endothelial progenitor cells (EPCs) from the bone marrow and improve cytotoxic drug delivery by normalizing the chaotic and abnormal architecture of tumor vessels and reducing vascular permeability. Consistently, several antiangiogenic therapies targeting the VEGF/VEGFR2/KDR signaling axis have shown to be effective in mouse models of NETs. In particular, a monoclonal antibody that blocks VEGF-A ligand (AF-493-NA) and a blocking antibody of the VEGFR2 (DC101) has been tested in the RIP-Tag2 mouse model of insulinoma with consistent antiangiogenic effects in microvessel density, endothelial cell proliferation, and antitumor activity with increased apoptosis [3, 15]. Bevacizumab, a humanized monoclonal antibody that recognizes and blocks VEGF (Fig. 3.1), failed to inhibit growth NETs cells in vitro, but reduced their angiogenic potential by blocking the cells’ ability to stimulate endothelial cell tube formation and proliferation and impaired tumor growth in animals [16].
Clinically, the activity of bevacizumab in NETs was tested in a randomized phase II study [17]. Forty-four patients on stable doses of octreotide were randomly assigned to 18 weeks of treatment with bevacizumab or PEG interferon alfa-2b. At disease progression (DP) or at the end of 18 weeks (whichever occurred earlier), patients received bevacizumab plus PEG interferon until progression. In the bevacizumab arm, four patients (18 %) achieved confirmed partial response (PR), 17 patients (77 %) had stable disease (SD), and one patient (5 %) had PD. No objective responses were observed in PEG interferon arm. Progression-free survival (PFS) rates after 18 weeks of monotherapy were 95 % in bevacizumab arm versus 68 % on the PEG interferon arm. Bevacizumab therapy also resulted in a significant reduction of tumor blood flow measured by functional CT scans.
A larger randomized phase III in patients with unresectable metastatic or locally advanced carcinoid tumors comparing depot octreotide acetate and interferon alfa-2b versus depot octreotide acetate and bevacizumab is being conducted since 2007 (SWOG S0518, clinicaltrials.gov NCT00569127). The results of this study are awaited in the near future.
Bevacizumab has also been tested in combination with cytotoxic drugs. Kulke et al. explored the efficacy and safety of the combination of bevacizumab plus temozolomide in a small phase II trial [18]. The combination showed an objective response rate of 24 % in pancreatic NETs but 0 % in carcinoid tumors. A phase II study of capecitabine, oxaliplatin, and bevacizumab for metastatic or unresectable NETs was reported in 2010 ASCO Annual Meeting. PR was observed in 7 pts (23 %), SD in 22 pts (71 %), and PD in 2 pts (6 %). Of the patients who achieved a PR, 6 had pancreatic NETs [19]. The combination with FOLFOX (oxaliplatin, leucovorin, and 5-fluorouracil) has also been tested with similar results [20]. Recently, a new phase II trial has been reported using the combination of bevacizumab with capecitabine in 49 patients with intestinal NETs. Nine (18.4 %) PR and 34 (69.4 %) SD were observed [21]. Another phase II trial testing bevacizumab plus traditional chemotherapy 5-FU/streptozotocin achieved an encouraging 55 % of PR with an acceptable toxicity profile [22]. Further phase III trials are warranted to establish the efficacy of adding bevacizumab to chemotherapy in NETs.
Other Vascular Players: PDGFR Axis and the Pericytes
Not only vascular cells are important for angiogenesis, but also the periendothelial support cells of the microvasculature or pericytes have shown to be relevant targets for effective antiangiogenesis. These cells mediate the stabilization of the vessels based on the synthesis of new basement membrane and tight association with endothelial cells; thus, endothelial cells can induce pericyte recruitment to protect themselves from death consequent to the lack of the crucial tumor-derived survival signals conveyed by VEGF [23]. Molecularly, a specific cross talk between endothelial cells and pericytes that implicates VEGF and PDGF is key for the vascular formation and maintenance and creates a crucial therapeutic opportunity that has been exploited [24]. For its supportive cooperative function aiding the endothelial cell stabilization and function, PDGFR inhibition has been developed in the context of dual inhibition of VEGFR and PDGFR [9]. Indeed, experimental studies with the RIP-Tag2 transgenic mouse model demonstrate a significant synergy when both endothelial cells and pericytes are dually blocked with VEGFR and PDGFR small molecule inhibitors such as sunitinib, which elicits detachment of pericytes and disruption of tumor vascularity in multiple stages in tumorigenesis, most notably in the often-intractable late-stage solid tumor [25, 26]. Although these positive results of the dual targeting of VEGFR and PDGFR, undesirable effects could emerge because a severe reduction or lack of pericyte coverage may disrupt the integrity of the vasculature, enabling tumor cells to transit into the circulatory system, thereby facilitating metastasis [23, 27].
On the clinical side, PDGFRs have been characterized in human pancreatic NET samples. PDGFR-α and PDGFR-β are commonly expressed both on tumor cells and tumor stroma [28]. The clinical approach to dually inhibit both VEGFR and PDGFR in NETs has been developed using several small molecule compounds such as sunitinib, sorafenib, vatalanib, and pazopanib (Fig. 3.1).
Sunitinib is the only antiangiogenic drug tested in a randomized phase III placebo-controlled trial [29] in patients with progressive well-differentiated pancreatic NETs, which is statistically positive in progression-free survival (11.4 months in sunitinib arm vs. 5.5 months in placebo arm). Sunitinib 37.5 mg/day was administered orally in a continuous schedule. The objective response rate was 9.3 % in the sunitinib group versus 0 % in the placebo group. This study was the first positive phase III trial with antiangiogenic drugs in the field and has changed the daily clinical practice in NETs. In a previous phase II study, 107 patients (41 carcinoid tumors and 66 pancreatic NETs) with documented disease progression were treated with repeated six-week cycles of sunitinib 50 mg/day, four weeks on and two weeks off. The overall objective response rate was 16.7 % in pancreatic NETs and 2.4 % in carcinoid tumors [30].
Sorafenib is an orally active, multikinase inhibitor with selectivity for the VEGFR-2, VEGFR-3, PDGFR-β, FLT3, c-kit, RET and RAF kinases. Sorafenib monotherapy has been evaluated in a phase II trial in 93 patients with NETs. The overall response rate was 10 % in both pancreatic and carcinoid NETs [31].
Vatalanib inhibits all known VEGFRs, with particular selectivity for VEGFR-2. At higher concentrations, vatalanib also inhibits PDGFR- β and c-kit. Two phase II studies were reported in 2008 in NETs, but both showed no significant radiological responses [32, 33]. Finally, pazopanib, another potent inhibitor of VEGFR, PDFGR-α/β, and c-kit, has been tested in 33 patients, most of them previously treated with mTOR inhibitors or other antiangiogénica drugs, with a 6 % of PR 79 % SD. This trial may introduce the concept of treatment sequencing with novel targeted agents in NETs [34]. Pazopanib has also been tested in combination with octreotide LAR in pancreatic NETs with 17 % of PR and a PFS of 11.7 months [35]. Axitinib, another potent inhibitor of VEGFR 1-3, PDFGR-β, and c-kit, is also been tested in this field.
Antiangiogenic Resistance
Clinical results using antiangiogenic drugs demonstrate only moderate gains in time to progression, and scarce benefits in overall survival, despite the long-term treatment. Why are there such modest and short-lasted benefits of antiangiogenic therapies in the clinic? The initial hypothesis was that antiangiogenesis therapy would not induce resistance (it would be “resistant to resistance”) because it targeted endothelial cells instead of the tumor cell itself [36]. Nevertheless, clinical and experimental evidence indicates that a vascular regrowth in tumors is present after reversal of VEGF inhibition [37]. In some cases, there is a period of benefit followed by progression and mortality that reflects an adaptive response by tumors. Tumors can manifest an “evasive response” by upregulating alternative pro-angiogenic signals (such as ephrins or angiopoietins), recruiting pro-angiogenic inflammatory cells or pericytes, accentuating invasiveness of tumor cells into local tissue to co-opt normal vasculature, and increasing metastatic seeding and tumor cell growth in lymph nodes and distant organs. By contrast, patients for whom there is no tangible benefit at the beginning of the therapy indicate that an intrinsic resistance to angiogenesis inhibitors exists [38].
VEGF inhibition produces vascular trimming and hypoxia, which leads to upregulation of multiple pro-angiogenic molecules, including VEGFs, FGFs, and angiopoietins, which can contribute to eventual resistance [3, 38]. Tumor hypoxia could select for tumor populations able to grow in low oxygen environments [39, 40] and/or provide alternate compensatory pro-angiogenic pathways to allow persistent neovascularization [41] Furthermore, studies in the RIP-Tag2 model have described progression of NETs in course of antiangiogenic therapies targeting the VEGF/VEGFR signaling axis. Thus, genetic or pharmacological potent angiogenesis inhibition can alter the natural history of tumors by triggering resistance to therapy and increasing invasion and lymphatic or distant metastasis [42, 43]. Similar results have been observed in other models [44].
Acquired resistance can also be developed due to rapid vascular remodeling of tumor-associated vessels as a consequence of antiangiogenic therapy. The mature remodeled vessels are resistant to antiangiogenic drugs, which usually target relatively immature vessels [45, 46].
Strategies to overcome this resistance mechanism are warranted. Based on preclinical data, several authors have proposed some strategies to overcome the antiangiogenic resistance that are based in combinatorial targeting of the VEGF pathway with other “escape” pathways that could be used for resistance (Table 3.1). In particular, some of these strategies, such as dual-targeted therapies, have been tested in xenografts [47]. The combination of bevacizumab and HIF-1 or Sp1 inhibitors may increase the therapeutic efficacy of antiangiogenic treatment [48, 49]. In another study, Allen et al. [50] suggest that co-targeting of VEGF and FGF signaling pathways can improve efficacy and overcome adaptive resistance to VEGF inhibition in the RIP-Tag2 model of pancreatic NETs. They tested the dual-FGFR/VEGFR tyrosine kinase inhibitor brivanib in both first and second line following the failure of anti-VEGFR2 antibody (DC101) or sorafenib showing promising results in overcoming resistance to VEGF-selective therapy.
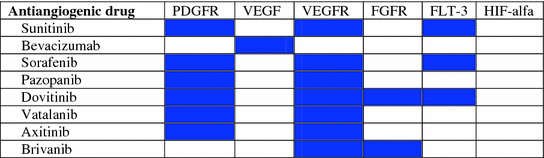
Table 3.1
Multi-target inhibitory profile of antiangiogenic drugs to address resistance

On the clinical side, some phase II studies have tested the combination of antiangiogenic drugs. 2-Methoxyestradiol (2ME2) administered in combination with bevacizumab has been evaluated in a prospective study in thirty-one patients with metastatic carcinoid tumors [51]. No confirmed radiological responses by RECIST were observed. However, 68 % of the radiologically evaluable patients experienced at least some degree of tumor reduction, and the median PFS time was 11.3 months. The results of a study [52] with the combination of sorafenib and bevacizumab were reported in 2011 ASCO Annual Meeting. The overall response ratio was 9.8 %, and the disease control rate at 6 months was 95.1 %. Median progression-free survival was 12.4 months. The most common grade 3–4 toxicities were hand–foot syndrome and asthenia, which occurred in 20.5 % and 15.9 % of patients, respectively. Another trial has tested the combination of bevacizumab and everolimus in NETs. Addition of everolimus to bevacizumab was associated with further decrease in tumor blood flow (15 %; p = 0.02) than bevacizumab alone. By intention-to-treat (ITT) analyses, there were 26 % of PR and 27 % of SD. The median PFS was 14.4 months [53]. Recently, preliminary results of another phase II trial with the combination of bevacizumab and temsirolimus, another mTOR inhibitor, have been reported. Confirmed PR was documented in 11 of the first 25 (44 %) evaluable patients [54].
On the other hand, the identification of biomarkers for response or resistance to a particular antiangiogenic regimen is imperative in order to monitor the efficacy of antiangiogenic therapy. A study in the RIP-Tag2 model of pancreatic NETs described that tumors refractory to therapy following long-term treatment with a vascular endothelial growth factor receptor-2 blocking antibody contained blood vessels with a prolific investment of pericytes expressing α-smooth muscle actin. This is a response of resistant tumors to the therapy, which is impairing neovascularization and/or eliciting vascular regression, and in order to maintain a core of preexisting blood vessels alive and functional, they increase the amount of pericytes [23]. Further studies are warranted to validate the occurrence of pericytes expressing α-smooth muscle actin as a biomarker for tumors refractory to therapy [55].
A Perspective
Morphological, histological, and molecular features of NETs strongly support the notion that angiogenesis is a promising target in these malignancies. Indeed, several antiangiogenic drugs have been clinically validated, and two of those have been recently approved and are being incorporated in the daily clinical practice of pancreatic NETs. Nevertheless, not all patients respond to these therapies, demonstrating upfront refractoriness to therapy or intrinsic resistance. This patient population has to be carefully studied and detected in the future to find the most appropriate patient selection marker or characteristic in order to effectively treat these refractory patients. On the other hand, antiangiogenic drugs demonstrate clinical efficacy in many NETs patients, but these clinical benefits are overshadowed by apparent acquired resistance to antiangiogenic therapies emerging in NETs. Therefore, overcoming antiangiogenic resistance is a crucial step in the future development of antiangiogenic therapies. Several strategies have been postulated to fight resistance, including multi-pathway inhibitors or multi-combination of antiangiogenic drugs that target different pathways that can revert resistance caused by the upregulation of alternative pro-angiogenic signaling molecules, the recruitment of vascular progenitor cells or pericytes to the forming blood vessels, and also in order to fight against the increased capabilities for invasion without angiogenesis observed in some animal models. In this sense, clinical studies that investigate and address these approaches in the coming years are warranted.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree