CHAPTER 10 Renal arteries and veins
Arteriography and venography (online videos 10-1 and 10-2)
Evaluation of the renal arteries begins with abdominal aortography to detect renal artery ostial disease and accessory arteries before selective catheterization is done. A 4- or 5-French (Fr) pigtail catheter (or similar configuration) is positioned with the side holes at the level of the main renal arteries (approximately the L1 or L2 vertebral body). Images are routinely obtained in an anteroposterior or slight right posterior oblique views.1 If computed tomography (CT) or magnetic resonance (MR) angiograms are available, they will guide selection of an optimal projection to place the renal artery origins in profile or may replace the catheter aortogram.
In patients with renal dysfunction (serum creatinine level greater than 1.2 to 1.5 mg/dL), various measures are taken to minimize the risk of contrast-induced nephropathy2–6 (see Chapter 2).
Anatomy
Development
In the fetus, the kidneys lie in the pelvis and receive blood from neighboring vessels, including the median sacral and common iliac arteries.7 With time, lateral splanchnic branches of the aorta begin to perfuse structures arising from the mesonephric ridge: adrenal glands, gonads, and kidneys. With the ascent of the kidneys to the midabdomen, they are ultimately supplied by the more caudal of these lateral splanchnic arteries. Anomalies in the location and number of renal arteries can be explained by incomplete regression of some of these primitive vessels.
Normal anatomy
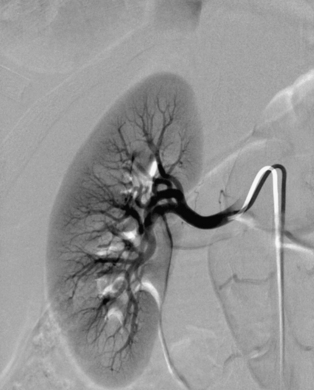
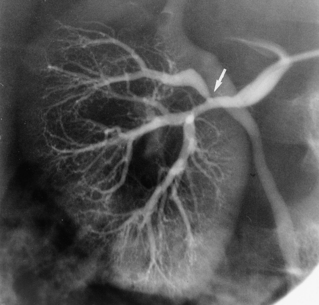
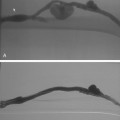
The renal arteries arise from the lateral surface of the aorta at about the L1-L2 vertebral level.8,9 The right renal artery runs posterior to the inferior vena cava (IVC) and right renal vein to enter the renal hilum. The left renal artery passes behind the left renal vein. The proximal renal arteries have small inferior adrenal, ureteric, and capsular branches, which often are not seen on imaging studies. At the renal hilum, the artery bifurcates into dorsal and ventral rami (Fig. 10-1). These trunks separate into segmental branches, which then further divide into lobar branches supplying the renal pyramids. The vessels successively branch into the interlobar, arcuate, and interlobular arteries. In the renal cortex, the interlobular arteries divide into afferent arterioles that supply the glomeruli.
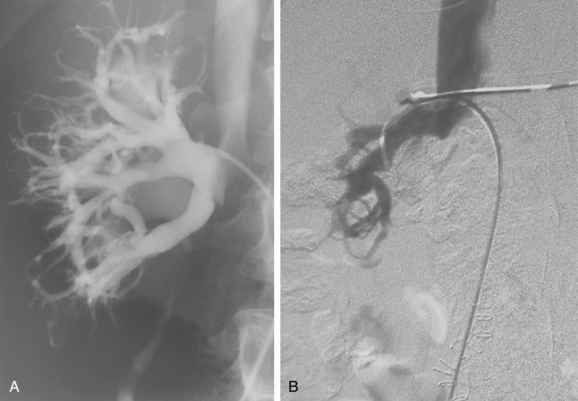
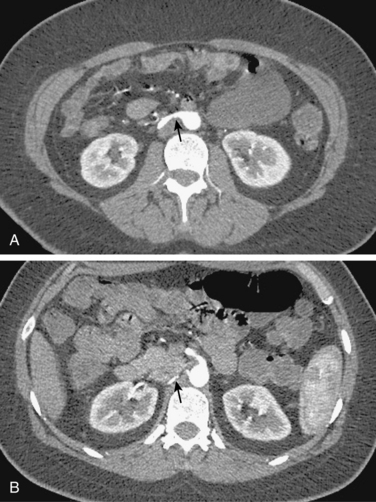
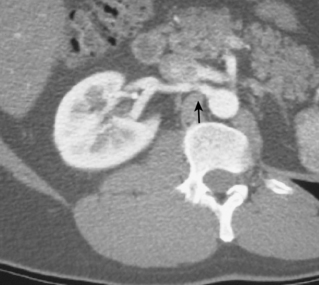
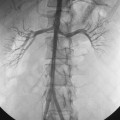
The renal venous system follows a branching pattern similar to that of the renal arteries. However, wide anastomoses exist among the intrarenal veins (Fig. 10-2). Both main renal veins run in front of their corresponding arteries. The right renal vein passes behind the duodenum and enters the right lateral surface of the IVC.10 The left renal vein, which is about three times as long, runs between the aorta and superior mesenteric artery and then drains into the left lateral wall of the IVC. The left gonadal vein enters the left renal vein along its undersurface just to the left of the spine (Fig. 10-3). The left adrenal vein enters the left renal vein on its superior surface usually in a combined trunk with the left inferior phrenic vein. The right adrenal and gonadal veins typically have separate entrances into the IVC at or near the origin of the right renal vein (see Figs. 13-8, 13-9, and 13-13). In most patients, branches of the ascending lumbar and hemiazygous venous systems enter the left renal and left gonadal veins (see Fig. 16-4). Some patients have valves in the renal veins between the hilum and the IVC.
Variant anatomy (online cases 4 and 31)
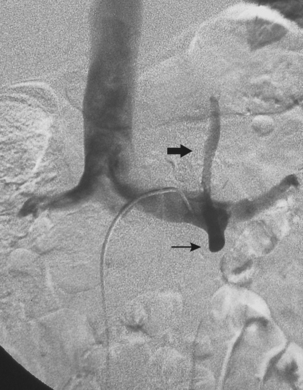
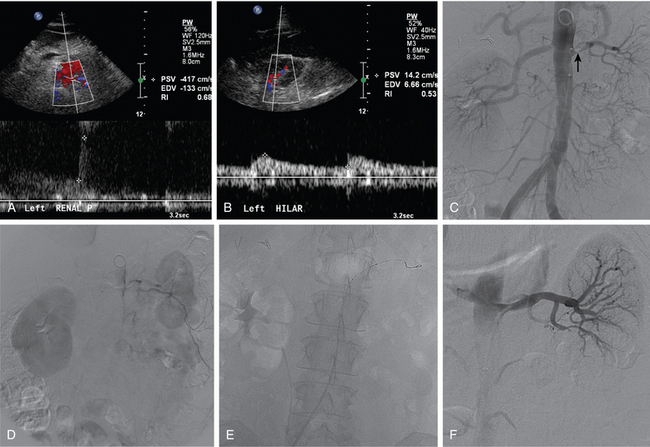
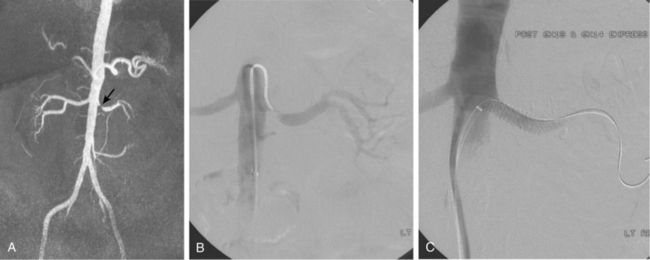
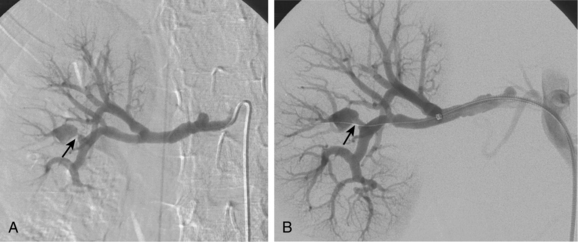
Accessory renal arteries supply one or both kidneys in about 25% to 35% of the general population.11–15 Most accessory renal arteries supply the lower pole of the kidney but may originate anywhere from the suprarenal aorta to the iliac artery (Figs. 10-4 through 10-6). Anomalies of position (i.e., ectopia), fusion, or rotation of the kidney are associated with variations in the origin and number of renal arteries. The horseshoe kidney usually is fed by three or more arteries arising from the aorta, iliac arteries, or both.
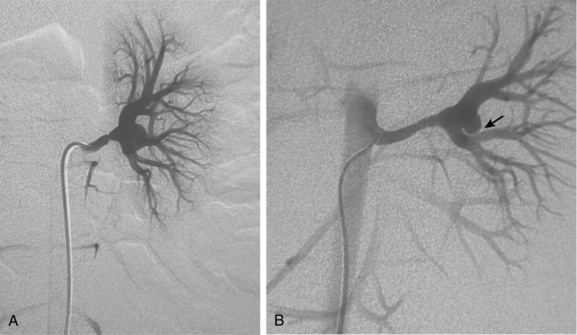
Several renal vein anomalies may be encountered16–19 (Table 10-1 and Figs. 10-7 through 10-9). That close to one third of the population has multiple (especially right) renal veins is not well appreciated by many interventionalists. The circumaortic left renal vein consists of a “preaortic” segment that enters the IVC in the usual location and a “retroaortic” segment between the kidney and the low IVC. The two veins may or may not communicate in the renal hilum. The solitary retroaortic left renal vein is a less common variant.15
Table 10-1 Renal Venous Anomalies
| Type | Reported Frequency (%) |
|---|---|
| Multiple right renal veins | 8 to 24 |
| Circumaortic left renal vein | 2 to 10 |
| Retroaortic left renal vein | 2 to 7 |
| Right gonadal vein enters right renal vein | <10 |
Collateral circulation
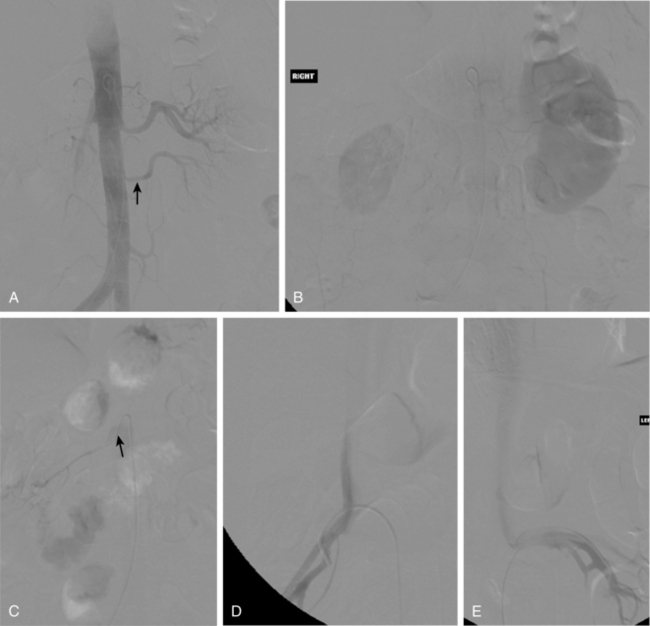
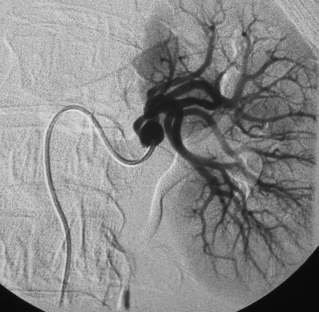
With renal artery stenosis or occlusion, an extensive collateral circulation maintains blood flow to the kidney. Although renal arteries have been described as end arteries, communications between extrarenal arteries and segmental, interlobar, and arcuate vessels do exist. The classic description of collateral arterial flow in the kidney was made by Abrams and Cornell.20 The intrarenal vessels are primarily supplied by three collateral networks: the capsular, peripelvic, and periureteric arteries. These three systems are ultimately fed by the lumbar arteries, aorta, internal iliac artery, inferior adrenal artery, and other vessels (Fig. 10-10).
In main renal vein obstruction, venous outflow occurs through ureteral, gonadal, adrenal, ascending lumbar, and capsular venous pathways (Fig. 10-11).
Major disorders
Atherosclerotic renovascular disease (online case 18)
Etiology and natural history
The renal arterial system plays a critical role in many patients with hypertension and chronic kidney disease. More commonly, long-standing or inadequately treated high blood pressure (from any cause) leads to progressive thickening and luminal narrowing of the small arteries of the kidneys. This nephrosclerosis is characterized by global damage of distal intrarenal vessels, which become irregular, tortuous, and pruned (Fig. 10-12). Less commonly, main, accessory, or branch renal artery obstruction (stenosis or occlusion) leads to so-called renovascular hypertension (RVH). The consequent reduction in intrarenal arterial pressure is sensed by the juxtaglomerular apparatus of the afferent arterioles. The renin-angiotensin-aldosterone system is triggered, leading to increased renin production, vasoconstriction of systemic arteries, sodium and water retention, and systemic hypertension. Other factors that sustain hypertension include endothelin release and upregulation of the sympathetic nervous system.21 Over time, untreated RVH will also cause nephrosclerosis that in and of itself contributes to elevated blood pressure. RVH is reported in about 3% to 5% of cases of hypertension in the general population, although this figure is hotly debated.22
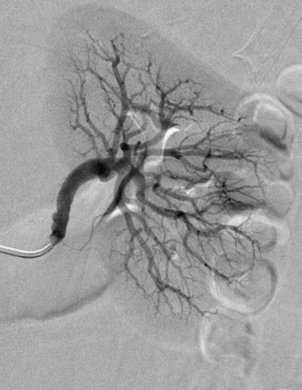
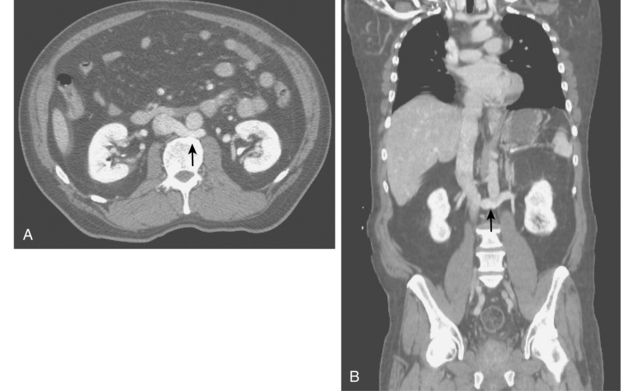
Figure 10-12 Nephrosclerosis of the left kidney. Note irregularity and pruning of distal intrarenal branches (compare with Fig. 10-1).
Ischemic nephropathy is generally defined as loss of renal function related to hypoperfusion from renal artery disease.23 Atherosclerotic renal artery disease affecting both renal arteries or the artery to a solitary kidney is a common cause of ischemic nephropathy. Bilateral occlusions may eventually cause end-stage kidney disease. However, diminished central blood flow may be a minor factor in development of chronic kidney disease compared with microvascular changes caused by diabetes, hyperlipidemia, or hypertension itself.24 Finally, the magnitude of renal artery stenosis has no direct correlation with the severity of kidney disease, unless the artery is completely occluded.25,26
Atherosclerosis is responsible for at least two thirds of cases of clinically significant renal artery stenosis.21 Obstruction usually results from aortic plaque engulfing the renal artery ostium (i.e., within 5 to 10 mm of the aortic lumen). Less frequently, plaque develops independently in the truncal portion of the renal artery. In several studies, atherosclerotic renal artery stenosis was found to be progressive in more than one third of cases.27,28 However, conversion to frank thrombosis is uncommon. Atherosclerotic renal artery disease must be distinguished from other causes of renal artery obstruction, particularly fibromuscular dysplasia (FMD; see later) (Box 10-1). Other kidney-related conditions that occasionally lead to chronic hypertension include trauma (e.g., Page kidney), cystic disease, renal cell carcinoma (RCC) or pheochromocytoma, renal artery aneurysm, reninoma, and renal infarction.
Clinical features
Most patients with atherosclerotic RVH or ischemic nephropathy are middle aged or older adults and have one or more risk factors for atherosclerosis. Younger patients are more likely to suffer from one of the less common causes of RVH (see later discussion). Although African Americans suffer from essential hypertension more frequently than do whites, RVH is relatively more common in whites.29 Patients with thrombosis superimposed on underlying disease often are asymptomatic. However, worsening of hypertension or loss of renal function may occur when there is occlusion of the artery to a solitary functioning kidney.
In the United States, the prevalence of significant renovascular disease (detected by duplex sonography) among individuals older than 65 years is about 7%.30 Significant atherosclerotic renal artery stenosis is associated with particularly high rates of chronic kidney disease (about 25%), coronary artery disease, peripheral arterial disease, and stroke.31
Imaging
Because hypertension is common but RVH is not, screening should be reserved for patients at moderate to high risk for RVH. Box 10-2 lists the criteria proposed by the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.32 Revised guidelines are currently in preparation.
Box 10-2 Signs of Renovascular Hypertension
ACE, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker
Color doppler sonography
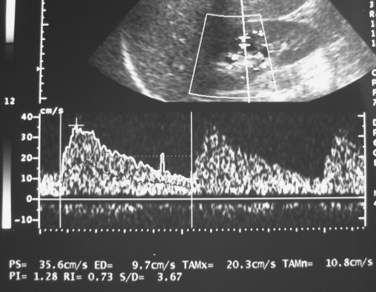
Ultrasound is one of the principal tools for detecting RVH because it is quick, relatively inexpensive, and completely safe (Fig. 10-13). If the aorta and main renal artery can be imaged, criteria for significant renal artery stenosis include intrastenotic peak systolic velocity (PSV) of greater than 180 cm/sec and PSV renal/aortic ratio of greater than 3.0 to 3.533,34 (Fig. 10-14). If these vessels cannot be seen because of technical factors, the intrarenal arteries are interrogated. With significant renal artery stenosis, damping and slowing of the time to peak systole (“parvus et tardus” waveform) is typical.35 In addition, flow acceleration is diminished and the acceleration time prolonged (<300 to 390 cm/sec2 and >0.06 to 0.07 sec, respectively).33,36 The resistive index([PSV − diastolic velocity]/PSV) usually is less than 0.45.34 However, duplex sonography is highly operator dependent, and some studies have failed to confirm the touted accuracy of this technique.37,38
Computed tomography and magnetic resonance angiography
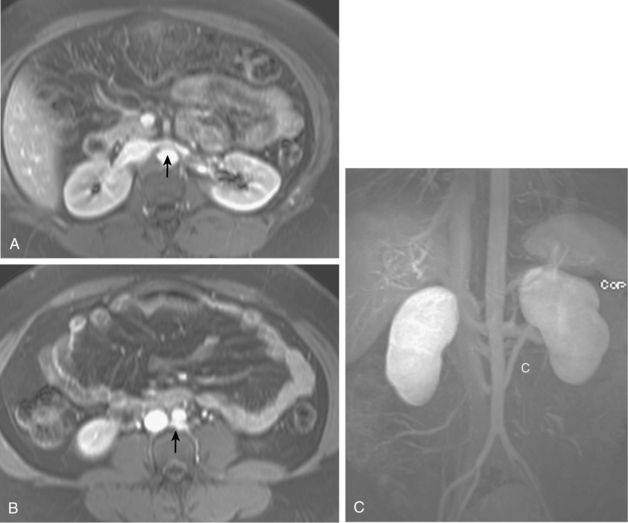
Many centers rely on gadolinium-enhanced MR angiography for screening patients with suspected RVH if renal function is close to normal. Patients with preexisting renal insufficiency are at risk for nephrogenic systemic fibrosis after receiving some gadolinium-based contrast agents. With current techniques, MR angiography can detect main renal artery stenoses with up to 90% to 100% sensitivity and 75% to 100% specificity38–45 (Figs. 10-15 and 10-16). Weaknesses of the method include identification of disease in accessory and segmental renal arteries and artifacts related to metallic clips, intravascular stents, or patient motion. However, isolated significant stenoses of accessory arteries in this population are uncommon (<2%).46
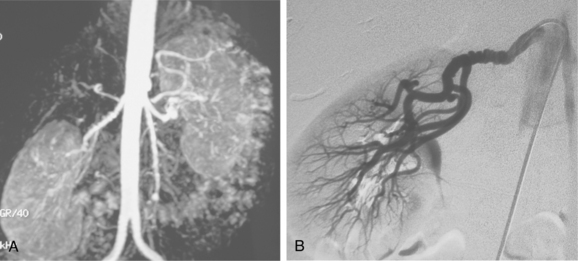
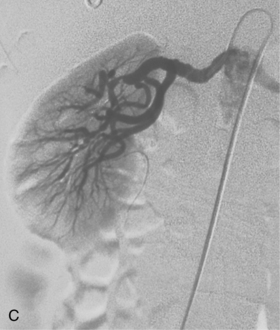
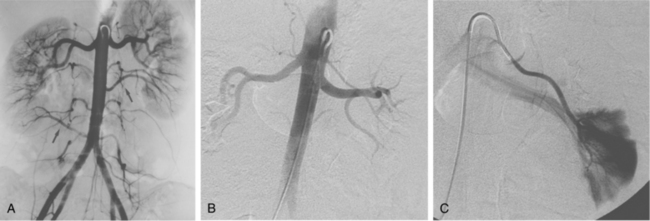
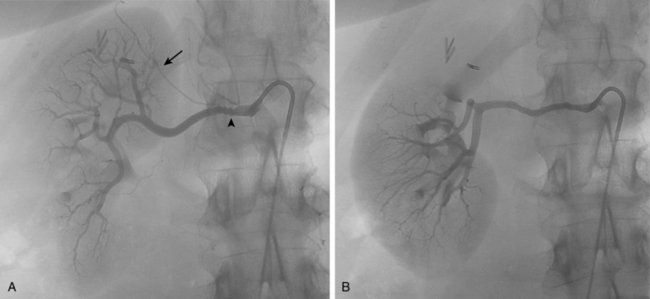
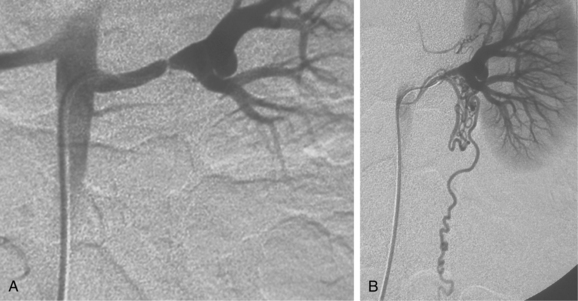
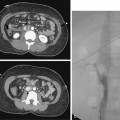
Figure 10-16 Medial fibroplasia type of fibromuscular dysplasia of the right renal artery. A, Magnetic resonance angiogram shows classic “string of beads” appearance of distal portion of the artery. B, Catheter angiogram confirms these findings. Mild changes of fibromuscular dysplasia are noted beyond the main artery. A systolic pressure gradient of 50 mm Hg was found. C, Following angioplasty with a 6-mm balloon, the gradient was reduced to 5 mm Hg.
Multidetector row CT angiography achieves comparable sensitivity and specificity to MR angiography in depicting renal artery stenosis39,40,44,47 (Fig. 10-17). Sixty-four–detector row CT is almost equivalent to catheter angiography in uncovering in-stent restenosis (which is obscured in MR angiography).48 Its major disadvantage is the need for iodinated contrast in a population at increased risk for contrast nephropathy.
Captopril renal scintigraphy
To a great extent, this functional rather than anatomic imaging modality has fallen out of favor in routine evaluation of patients with suspected RVH.40,49
Renal vein renin sampling
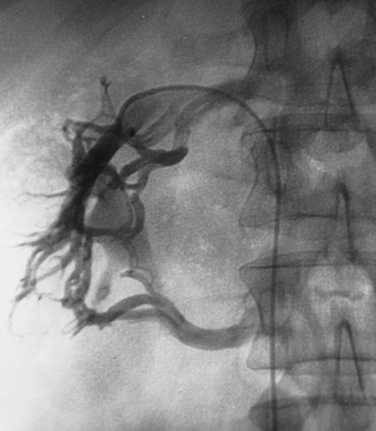
Normally, the ratio of renin activity in the renal vein versus the IVC is about 1.24.50 In a patient with RVH and two functioning kidneys, the affected kidney overproduces renin, and renin secretion from the contralateral kidney is suppressed. Several criteria have been used to diagnose RVH based on renal vein renin values, including a renal vein renin ratio between the involved and uninvolved kidney of greater than 1.5 and a ratio of (renal renin–IVC renin)/IVC renin of greater than 0.48 (Vaughan formula).51 While renal vein renin sampling was once a mainstay of RVH diagnosis, it is seldom used by interventionalists (Fig. 10-18).
Catheter angiography
Catheter angiography is still the gold standard for the diagnosis of RVH. Initial abdominal aortography may not be necessary if high-quality CT or MR angiography studies clearly depict the renal artery ostia and identify all renal arteries. Narrowing of accessory renal arteries or intrarenal branches can cause RVH and should be carefully sought; intrarenal stenoses are particularly common in children with RVH (Figs. 10-19 and 10-20). Stenoses may go undetected for several reasons, including oblique origin of the renal artery from the aorta and superimposition of intrarenal branches. Multiple angiographic views may be necessary to identify such hidden lesions.
Atherosclerosis produces irregular narrowing of the renal ostium or proximal renal artery (see Figs. 10-14 and 10-15). Bilateral disease is common, and infrarenal aortic atherosclerosis usually is present. After a stenosis has been identified, its hemodynamic significance must be proved by one of the following criteria21,52:
Treatment
Selection of patients with RVH or ischemic nephropathy for endovascular or operative therapy is highly controversial21,53 (Box 10-3). Recent improvements in anti-hypertensive medications and routine lifelong treatment of this population with lipid-lowering “statins” and oral antiplatelet agents may allow many people with RVH and normal or mild renal insufficiency to be treated medically. The mere presence of a significant renal artery stenosis does not by itself warrant treatment, because not all such patients will have a positive clinical outcome from arterial recanalization. In addition, the procedure itself may worsen renal function by contrast nephropathy, atheroembolization, or, rarely, complete vessel occlusion.
Box 10-3 Indications for Endovascular Atherosclerotic Renal Artery Treatment
ACE, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker
Renal artery stent placement
Over the past two decades, endovascular treatment of renal artery disease has been embraced by interventionalists of all kinds.54 Advocates point to numerous large historical series that support the value of renal artery stents in treating hypertension or stabilizing (or even reversing) renal insufficiency.55–58 However, this enthusiasm has been tempered by several recent randomized trials that have failed to show significant benefit, particularly in light of nontrivial adverse events (see later discussion). Although some of these studies are fraught with important drawbacks, many physicians outside the interventional community are skeptical of routine application of this aggressive approach.
Patient selection
Box 10-3 lists the primary indications for intervention in patients with atherosclerotic RVH or ischemic nephropathy.21,59 Angioplasty alone is rarely employed in this setting, with the possible exception of focal truncal stenoses.60 Stents significantly improve the technical success of renal artery angioplasty for atherosclerotic disease, particularly for ostial lesions (from 55% to 70% to more than 95%).56,60–65 This improvement alone may largely explain the better long-term patency rates associated with stent placement. Use of stents in renal arteries with nominal diameter of less than 5 mm usually is avoided because of the relatively higher rates of clinically significant restenosis in this setting.66
Technique (see fig. 10-14)
Most interventionalists continue the patient’s blood pressure medication until the procedure is completed. The dose of iodinated contrast material should be kept to an absolute minimum. In patients with preexisting renal dysfunction, various measures are taken to protect the kidneys including use of carbon dioxide angiography and administration of N-acetyl cysteine and intravenous bicarbonate infusion (see Chapter 2 and Fig. 3-20). All patients should receive aspirin (325 mg orally) or clopidogrel (300 mg oral loading dose) beforehand. If there are no contraindications, the former is continued for the patient’s lifetime or the latter for at least 3 to 6 months afterward. Parenteral platelet inhibition may be helpful in minimizing deleterious effects to the kidney from atheroemboli or contrast.67,68 Full anticoagulation with heparin (or a direct thrombin inhibitor if heparin allergy history exists) is instituted before crossing the obstructive lesion. An intraarterial dose of nitroglycerin (150 to 200 mg) may help prevent guidewire or catheter-induced vasospasm.
The diagnostic catheter is replaced with a 5- or 6-Fr guiding sheath or catheter with distal curve appropriate for the renal artery origin. Traditionally, the stenosis was crossed with a floppy 0.035” guidewire, a 5-Fr catheter advanced beyond the lesion, and the balloon or stent inserted over a stiff 0.035” Rosen or TAD exchange wire. Most practitioners now favor low-profile platforms.69 Thus the stenosis is crossed with a steerable 0.014” to 0.018” microwire followed by a microcatheter. If difficulty is encountered traversing the lesion, several maneuvers should be tried. Changes in the patient’s respiration can dramatically alter the aortorenal artery angle. Stiffer (or sometimes less stiff) guidewires may be helpful.
Recent data prove that endovascular renal procedures reliably cause showers of atheroembolic particles into the distal vasculature.70,71 This embolic burden may negate any positive effect expected from the intervention. Distal embolic protective devices (occlusion balloons or permeable filters) that trap this material may prevent microembolic injury to the treated kidney. These filters are strongly advocated by some experts.
Results
Success in renal artery revascularization for RVH is judged by blood pressure response, with clinical benefit defined as cure (i.e., blood pressure lower than 140/90 mm Hg without medication) or improvement (i.e., reduced number of medications required to maintain blood pressure below 140/90 mm Hg or a 15-mm Hg reduction in blood pressure on the same or reduced number of medications).22 In ischemic nephropathy, clinical benefit is defined as reduction or stabilization in serum creatinine or flattening of the renal dysfunction curve (1/creatinine versus time).
A widely patent artery can be created with a metallic stent in greater than 95% of cases.24,61,63,72,73 In atherosclerotic RVH, actual cure of hypertension is achieved in less than 10% to 20% of individuals whether stents are used or not. Blood pressure control is better in about 50% to 80% of patients. Based on historical series, angioplasty and stent placement lead to improvement or stabilization of renal insufficiency in about 70% of cases; however, in some (but not all) series, that benefit diminished over time.64,66,72,74 The value of embolic protection is unproven despite some encouraging nonrandomized series.67,68,75
The reported angiographic restenosis rate for atherosclerotic lesions is about 20%.63,66,76,77 However, the reported Doppler sonographic restenosis rate at 9 to 12 months has ranged from 21% to 50%.78,79 Luminal compromise seems to increase slowly over time. One important predictor of clinically significant restenosis is small initial vessel diameter (<5 mm). Preliminary trials suggest that drug-eluting stents may be of added value in such small caliber arteries.80,81 Restenosis is treated by repeated balloon angioplasty without or with additional stent placement82,83 (see Fig. 1-10).
Despite encouraging results from retrospective studies and registries, controlled randomized trials of stent placement versus best medical therapy for patients with atherosclerotic renal artery stenosis have not supported routine endovascular therapy for management of RVH or ischemic nephropathy.24,53,73,84,85 Critics are quick to note the frequency of major adverse events associated with stent insertion, including loss of renal function after revascularization in 10% to 20% of individuals.21 These studies had numerous deficiencies, including inclusion of a substantial number of patients with nonsignificant renal artery stenosis and lack of standardization of stent insertion techniques. Nonetheless, the cumulative burden of these recent trials is sobering. There is hope that the ongoing multicenter CORAL and RADAR trials will satisfy both proponents and skeptics of renal artery stent placement.87,88
Complications
Major complications occur in about 8% to 15% of procedures22,89 (see Fig. 10-14). Permanent decline in renal function is noted in about 6% of cases and usually is caused by contrast nephropathy and/or microembolization. Periodic surveillance CT angiography or duplex ultrasound is advisable to detect restenosis and the need for further treatment.48,79
Surgical therapy
In patients with RVH or ischemic nephropathy, surgery is reserved for failures of endovascular treatment, cases with associated aortic disease that require open operation, and most renal artery occlusions.90 If minimal aortic disease is present, the standard procedure is aortorenal bypass grafting with autologous vein. In patients with severe aortic atherosclerosis or an aortic aneurysm, the hepatic or splenic artery may be used as a bypass conduit for the right or left renal artery, respectively.91,92 If the main renal artery is occluded but the distal artery reconstitutes through collaterals, bypass is sometimes done to improve function or treat RVH. Otherwise, hypertension may be treated by excision of the renin-producing kidney (see Fig. 10-18).
Renal artery fibromuscular dysplasia (online case 76)
Etiology and clinical features
Fibromuscular dysplasia (FMD) is a group of related noninflammatory disorders of unknown etiology in which affected arteries become narrowed, aneurysmal, or dissected.93 FMD is not rare; it is found in about 3% to 5% of preoperative imaging studies of potential kidney donors.94,95 Six types of FMD are described in one traditional classification scheme; the most common type is medial fibroplasia93,96 (Table 10-2). In this form of the disease, the normal media is replaced by fibrous tissue and extracellular matrix. The thickened segments alternate with sites of medial thinning, which produces the classic “string-of-beads” appearance of the lumen on imaging studies. The less common types of FMD cause luminal beading, smooth and tapered stenosis, focal bandlike narrowing, dissection, or aneurysm without stenosis.
Table 10-2 Classification of Subtypes of Renal Artery Fibromuscular Dysplasia
| Type | Frequency (%) | Morphology |
|---|---|---|
| Medial fibroplasia | 60 to 70 | Alternating narrowing and aneurysms (“string of beads”) |
| Perimedial fibroplasia | 15 to 25 | Irregular, beaded narrowing |
| Medial hyperplasia | 5 to 15 | Tubular, smooth narrowing |
| Medial dissection | 5 | False channel in media |
| Intimal fibroplasia | 1 to 2 | Focal, smooth narrowing |
| Periarterial fibroplasia | <1 | Tubular, smooth narrowing |
FMD is largely a disease of young or middle-aged women. However, it may be seen in men and young children. The disorder is often asymptomatic and only detected on imaging studies obtained for other reasons. Difficult to control hypertension and onset at a younger age should suggest the diagnosis. Renal FMD rarely results in ischemic nephropathy.
Imaging
The approach to imaging of patients with suspected FMD-related renovascular hypertension is similar to that for cases of atherosclerotic-associated disease (see earlier discussion). Most patients are screened with duplex sonography and then referred for CT or MR angiography if ultrasound is positive. The latter modalities approach but do not achieve the accuracy of catheter arteriography in the diagnosis of main renal artery FMD.97,98 Catheter angiography is still necessary to detect or exclude branch vessel involvement, so the threshold for performing an angiogram should be low when the index of suspicion is high.
FMD typically attacks the middle to distal renal artery and less commonly intrarenal branches. However, proximal (but rarely ostial) lesions do occur. The aorta usually is disease-free. Bilateral FMD is seen in about two thirds of cases; the right kidney is affected more commonly than the left.99 The classic appearance of the common medial fibroplasia type of FMD is a “string of beads” (see Figs. 1-25 and 10-16). The other forms of FMD have different morphologies (Figs. 10-21 and 10-22; see also Table 10-2). However, the pathologic subtype cannot always be predicted by the angiogram.100
Treatment
Renal artery angioplasty
By common consent, balloon angioplasty is the first-line treatment for symptomatic renal FMD (see Figs. 1-25, 10-16, and 10-21).
Technique
The distal portion of the balloon must be kept away from smaller branch vessels when it is expanded. FMD lesions may require multiple prolonged balloon inflations or pressures in excess of the 4 to 12 mm Hg needed to crack most atherosclerotic plaques. Cutting balloons (see Chapter 3) can be helpful in this situation.101 If the patient complains of severe pain, the vessel may be overstretched, and the balloon should be immediately deflated. Renal artery perforation is fortunately rare but may be remedied by prolonged balloon inflation or placement of a covered stent.
Angioplasty is considered successful when the residual stenosis is less than 30% and the systolic pressure gradient is less than 10 mm Hg. An irregular “ratty” luminal surface alone is not an indication for stent placement because these vessels often remodel over time. If results are inadequate, stent placement should be considered102 (see earlier discussion).
Results
Technical success occurs in greater than 90% of cases; this figure approaches 100% when stents are used as needed.102,103 Clinical benefit is noted in about 75% to 90% of cases, and results usually are long lasting. In fact, complete cure of hypertension from medial fibroplasia-type FMD is noted about half the time. However, these figures primarily apply to that common subtype, because large series that focused on the less common subtypes are unavailable. FMD-related renal artery aneurysms have been excluded with covered stents.104
Acute renal artery occlusion (online case 82)
Etiology and clinical features
Box 10-4 lists the primary reasons for complete renal artery occlusion.105,106 The most common etiology is an embolus, which originates from the heart in about 90% of cases.107 Thrombosis superimposed on existing atherosclerotic disease is another important cause. Blunt or penetrating abdominal trauma can produce an intimal tear, dissection, or complete avulsion of the renal artery, any of which can result in acute thrombosis. Renal artery dissection is usually an extension of the process arising in the abdominal aorta.