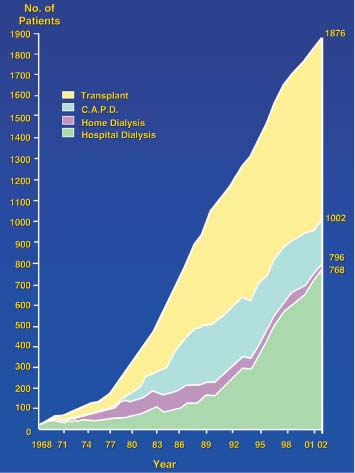
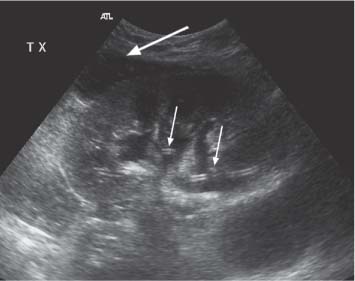
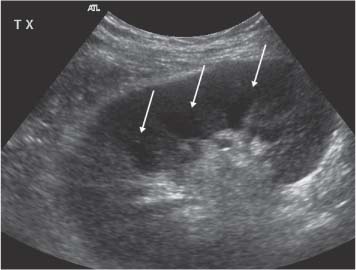
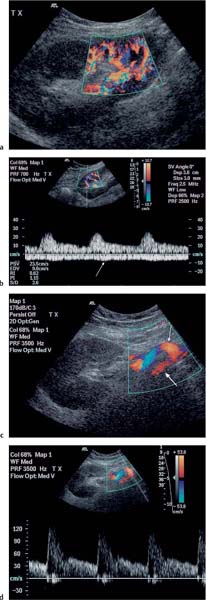
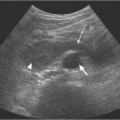
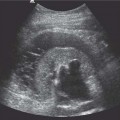
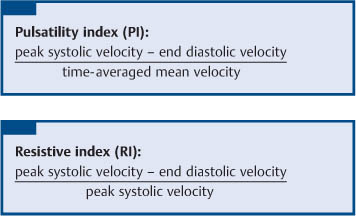
5 Renal Transplantation The road to what we now regard as the routine and successful procedure of renal transplantation has not been smooth and indeed dates back to the earliest days of Carrel’s experimental attempts at transplantation at the beginning of the 20th century, which resulted in the Nobel Prize of 1912.1 During the nonimmunosup-pressed attempts at transplantation in the 1950s and then the more successful and encouraging outcomes of the twin-to-twin transplants2 later that decade, there were so many severe setbacks and pitfalls that many groups at the time wondered whether further work in this area was justified. However, a better understanding of tissue rejection, the introduction of steroid and azathioprine in 1963,3 and then more specifically the use of cyclosporin A by Calne et al. in the 1970s,4 opened the door to progress. Fig. 5.1 Graph of the various forms of treatment of chronic renal failure in the West of Scotland, 1968–2002 Further improvements in surgical technique, new and more effective antirejection therapy which over the years has become less toxic, the routine use of ultra-sound in the 1970s and Doppler a decade later, and the rapid development of interventional radiological techniques all combined in a very synergistic manner to make this a routine technique with the successful clinical outcome we now take for granted. It is fair to say, however, that without the fortitude and dedication of these early pioneers, it is unlikely that this status would have been achieved and indeed much of the work that will be described in this chapter owes a great debt of gratitude to those early pioneering and informative years. The number of patients with end-stage renal disease (ESRD) continues to rise slowly and it is estimated that it will not plateau until before the middle of the 21st century. This largely reflects a combination of an aging population and a significant improvement in long-term prognosis in this patient group, particularly in those patients with co-morbidity diseases and in particular diabetes mellitus.5 The implication, therefore, for public health resources, in this group, is unremitting in terms of diagnosis, treatment, and long-term surveillance, and it is clear that this combination of factors is likely to constitute a serious health-resource issue.6 Treatment options for ESRD varies and includes hemodialysis and peritoneal dialysis; there is no doubt, however, that the treatment option of choice is renal transplantation (Fig. 5.1). Limitations of the latter option, however, are well-appreciated and well-recognized by the general public, as many public health campaigns highlight the continuing shortage of suitable donor kidneys.7 Thankfully better donor–recipient matching,8 use of more potent immunosuppressive regimes9in combination with improved surgical techniques have all resulted in an improvement in outcome, the one- and five-year graft survival rates in Europe in the mid-1990s being 77–82% and 57–64%, respectively.10 Further improvements in these figures can be expected through earlier recognition and treatment of a variety of transplant insults and complications, and indeed current figures show a one-year graft survival of 90% and a five-year survival of 70%, with slightly higher values for living donor kidneys, and slightly lower for second and subsequent transplants. (For current updated information see www.uktransplant.org.) It is universally agreed that transplantation is without doubt the treatment of choice. For a number of patients with chronic renal failure, a successful renal transplant will provide a glomerular filtration rate (GFR) of 50–60 mL/min, which although only half of the normal adult value, is sufficient to return the majority of patients to a normal and independent life pattern. Such an improvement in lifestyle and quality of life is difficult, and some would say impossible to measure or cost. However, what is clear is that in the potentially harsh world of health economics, the cost-benefit of a successful and functioning transplant far outweighs that of failure, and this is one reason why many resources are focussed and targeted on the pretransplantation, peritransplantation, and posttransplantation period to ensure as high a success rate as is possible. The average normal life expectancy of a transplant kidney is 7–10 years, increasing to 15–20 years when a live donor organ is used. Although many different imaging techniques are employed in dealing with the transplant patient, there is absolutely no doubt that ultrasound is central and crucial, and that it is very useful in both the early postoperative period as a noninvasive indicator of transplant dysfunction and also in the long-term follow-up of many of these patients. This chapter will highlight the value and application of this imaging technique. There are few contraindications to renal transplantation, and this treatment option should be considered for all patients with ESRF that is of a severity to require dialysis. Those unfit for transplantation are generally those who are unfit for anesthesia or surgery, for example severe cardiac or respiratory disease, or severe arteriopathy. In addition, the known potential problems of immunosuppression in the context of pre-existing infection and malignancy should be considered, as should the risk of disease recurrence, which is particularly associated in patients with oxalosis or active vasculitis. The main sources of organ transplantation are generally either brain-dead, ventilated organ donors or live, related donors. Although the majority of transplants in developed countries are cadaveric in origin and more live, related operations are performed in developing countries, there is no doubt that due to the current shortage of available organs there is a small but notable increase in live donor transplants now being recorded in developed countries. Indeed, both live, related and live, unrelated donor transplantation is now possible and has a very similar outcome and prognosis. Some technical differences exist in terms of surgical technique and recipient outcome between cadaveric and live, related donor transplants. However, the overall management of the recipient can be regarded as similar. In order to reduce the risk of rejection as far as possible, in particular hyperacute rejection, the detection of donor-specific antibodies in the lymphocyte cross-match test is a contraindication to transplantation. The risk of subsequent episodes of acute rejection is partially dependent upon the degree of human leukocyte antigen (HLA)matching between donor and recipient. The genes determining the HLAs are located on chromosome 6.11,12 The importance of HLA matching is reflected in the improved graft survival of a fully HLA-matched graft (T1/2, 17.3 years) compared with an incomplete, HLA-mismatched graft (T1/2, 7.8 years).13,14 The transplant procedure should be performed, certainly within 24 hours of organ retrieval and at worst 48 hours. During this period the recipient will have been chosen, appropriately prepared for theatre, i. e. screened for infection, cardiorespiratory reserve assessed, and additional dialysis given if fluid/metabolite imbalance requires correction. A live, related donor, who can either be a family member or close friend, is screened with a combination of clinical history, examination, and HLA status assessment. Other tests vary from center to center and include a 24-hour creatinine clearance, serology, liver function tests, and a choice of intravenous urogram, dimercaptosuccinic acid (DMSA) scan, renal arteriography, and computed tomography (CT) and/or magnetic resonance (MR) imaging to assess renal function. Traditionally the transplant renal vessels are anastomosed to the external iliac artery and vein in the case of a cadaveric transplant and to the internal iliac vessels in a live, related transplant. In those receiving a third transplant, an intraperitoneal approach to the iliac vasculature can be used if required. The vesicoureteric anastomosis is performed by joining a shortened donor ureter with the dome of the bladder. As a consequence of this procedure the lower end of the ureter, particularly if the ureter is left too long, may be prone to ischemic insult and may result in stricture formation, which can on occasion be functionally significant. At many centers a perioperative ureteric stent is placed; this is normally removed at three months (Fig. 5.2). Fig. 5.2 Normal functioning transplant kidney in the early postoperative period. The ureteric stent (arrows) can easily be seen curled up within the renal pelvis and running down the proximal ureter, which is only slightly distended. A small peritransplant collection (thick arrow) is noted. This was a benign postoperative collection which did not require any intervention Postoperative complications vary from center to center but include bleeding (< 1%), major vascular occlusion of an artery or vein (1–2%), wound infection (1.6–6.3%), and a number of urologically-related complications, including anastomotic leak, obstruction, and hematuria (1.3–7%).15–18 The aim of immunosuppression is clearly to prevent rejection without inducing infectious complications or serious drug toxicity. A number of options are now available. A conventional regime may have included a combination of cyclosporine (cyclosporin A), azathioprine, and steroids (prednisolone). However, newer agents, including tacrolimus,19 mycophenolate,20 and sirolimus (rapamycin), are also often used. Antibody therapies, i. e. anti-interleukin 2, are effective and less toxic than the older, more traditional humoral agents, including OKT3 and antithymic globulin, and are often used in high-risk patients. Clearly, with an increased number of options, immunosuppressive regimes will vary from center to center. The treatment of established acute rejection is normally with high-dose oral or intravenous steroids and in resistant cases immunodepletion with antibody therapy or tacrolimus. Unfortunately, there is no effective treatment for hyperacute or chronic rejection. Summary points: • The one- and five-year graft survivals are currently 90% and 70% • A combination of an aging population and increased requirements constitutes a major public health and ethical issue • There are few contraindications to transplantation • Disease recurrence posttransplantation is high in patients with oxalosis or active vasculitis • Immunologically well-matched kidneys have a favorable outcome The transplant kidney can normally be visualized in either iliac fossa, largely lying a number of centimeters below the skin surface and therefore easily accessible. Orientation of the transplant can be variable depending on the surgical technique; however, perpendicular images can normally be obtained. As for all ultrasonic techniques, resolution depends on a well-appreciated number of factors, including patient build, depth of transplant beneath the skin surface, and the presence or absence of postoperative edema. In general a 4-MHz probe gives a good overall assessment of the kidney, perirenal, or transplant collections and also allows interrogation of the transplant vasculature, including the more deeply situated iliac vessels. A higher frequency probe (e. g., 7 MHz) does give excellent near-field resolution and therefore good anatomical detail of the transplant kidney, but in all but the thinnest of patients will probably not allow visualization of the deeper situated vascular structures. Morphologically there is no difference between the transplant and native kidneys, which are identical, in that there is a well-defined renal parenchyma peripherally with a bright echogenic fat containing renal sinus centrally. Due to the improved resolution of the transplant kidney, the renal pyramids are more commonly visualized and are hypoechoic relative to the overlying parenchyma and are regularly spaced with no communication between them, this being a useful differentiating feature from calyceal dilatation (Fig. 5.3). It is not uncommon to observe mild hydronephrosis in the early postoperative period in the “normal” transplant kidney and this largely reflects postoperative edema with some ureteric compression at the vesicoureteric anastomosis. Although this may resolve with time, this may not always be the case. However, assuming renal function to be normal, a minor degree of dilatation is generally documented to act as a baseline on which subsequent examinations can be evaluated. It is important not to confuse the transplant vessels, i. e. the artery and vein at the renal hilum, with a dilated or prominent renal pelvis, and these should be easily differentiated with the use of color or power Doppler. The bladder should be routinely imaged when possible and should be echo-free. The presence of intravesical turbid echoes may indicate either hemorrhage or infection, depending on the clinical situation. Color Doppler ultrasound is extremely helpful as it provides an instantaneous assessment of the intrarenal vasculature and therefore a global impression of overall transplant perfusion and helps identify the transplant artery and vein and the iliac vessels. Although the color flow information in itself is purely qualitative, in many circumstances this information is both helpful and reassuring. As with all Doppler techniques, however, spectral Doppler analysis is required for quantification (Fig. 5.4a,b). The technique of color flow Doppler in the transplant has much in common with that used elsewhere, i. e. the vessel is first identified with color, the spectral gate placed over the vessel, and a Doppler tracing obtained. The spectral Doppler renal waveform is characteristically a low-resistance waveform, i. e. it has a “ski-slope” appearance, with diastolic flow normally contributing at least up to a third of the peak systolic value. Any reduction in diastolic flow may represent a pathological process. In order for color Doppler ultrasound to be used effectively in the early transplant period, serial studies are required until renal function is clinically satisfactory. A number of Doppler indices including the pulsatility index (PI), resistive index (RI), and the systolic-to-diastolic and diastolic-to-systolic ratios can all be used, although the commonly utilized indices are the PI and RI. There is no perceived advantage to using one over the other. Fig. 5.3 Normal transplant kidney. The renal pyramids (arrows) are regularly spaced and hypoechoic relative to the parenchyma. They do not communicate and are readily differentiated from calyceal dilatation The main transplant artery can be very difficult to visualize due to its tortuosity (Fig. 5.4c), and possibly partly because of this, a wide range of peak systolic values have been quoted for this vessel. At our center a cut-off value of 2.5 ms–1 is used,21–23 i. e. any value below this is regarded as normal, whereas above it is taken to represent a significant transplant artery stenosis. Examination of this vessel may be a time-consuming and exacting procedure due to the twists and turns previously described and the requirement of precise angle correction, and therefore accurate velocity readings cannot be overemphasized (Fig. 5.4d). No specific values exist for the transplant renal vein. However, often the prime consideration, at least in the early transplant period, is simply whether flow is absent or present. It is of paramount importance that both the iliac artery and vein are identified in order to distinguish them from the renal vessels and indeed to exclude a more proximal lesion in the iliac vessel, for example the iliac artery, which may contribute or indeed be the sole cause of adverse renal function. Summary points: • A 4 MHz ultrasound probe gives the best overall assessment of the transplant kidney • Mild hydronephrosis is “normal” in the early postoperative transplant period • Serial color Doppler ultrasound measurements are required to monitor transplant dysfunction • The PI and RI are the most commonly used Doppler indices; there is no advantage of one over the other • Both the transplant and iliac vessels should be identified to avoid confusion • The peak systolic velocity (PSV) in the transplant artery is normally < 2.5 ms-1 Early complications are numerous and varied, and include parenchymal insults, i. e. acute tubular necrosis (ATN), acute rejection, or both, vascular occlusion, obstruction, hemorrhage, urinary leak, collections, infections, and drug toxicity related to antirejection therapy itself. Often many of these complications may be differentiated on a combination of clinical history, bacteriology, and ultrasound. When all is said and done, often the main differential diagnosis is between acute rejection and ATN and this can be very difficult clinically as symptoms are generally absent. As both entities require a different approach to treatment, early and accurate diagnosis is essential. Despite many attempts to the contrary it has not been possible to differentiate between these entities with color Doppler imaging and a histological diagnosis is still required.24–27 Despite this limitation, ultrasound is still useful in its dual role of not only assessing and monitoring transplant dysfunction but also in assessing response to therapy. Acute tubular necrosis (ATN) is common in the early transplant period and up to 30% ofpatients may require dialysis in the early stages. Delayed graft function is rare in the live, related donor situation. ATN is principally related to both donor and donor kidney, particularly the warm ischemic time. In those patients with established ATN requiring dialysis, recovery usually occurs within one to two weeks of transplantation, although in rare cases it can be delayed for significantly longer periods of time ofup to three months. Fig. 5.4 a Normal color Doppler scan of the transplant kidney. The intrarenal vessels are well-visualized and extend to the periphery of the cortex. The arterial (red) and venous (blue) branches can be easily distinguished. b Normal spectral Doppler waveform from an interlobar artery. Having selected and drawn around an appropriate arterial waveform, the machine has automatically calculated both the PI and RI. Venous flow is noted beneath the baseline (arrow) c Normal color Doppler scan of the origin of the transplant renal artery (thin arrow) at its origin from the external iliac artery (thick arrow). d Spectral Doppler waveform of the transplant renal artery just distal to its origin. The normal renal artery waveform is well demonstrated and has a peak systolic velocity (PSV) of 1.20 ms–1 Diagnosis of acute rejection is by biopsy, which in the experienced hand is safe, with the complication of hemorrhage, requiring blood transfusion, and pain relief, requiring analgesia, of less than 5%.28 Significant complications (SMI) remain below 5% despite needle size. However, the recommended technique is using an automated core biopsy system under direct ultrasonic guidance.29–31 Needle sizes vary from 14–18-gauge (G). However, at our institution 16 G is normally employed. Acute rejection can affect up to 40% of patients and peaks at one to three weeks posttransplantation. Assuming it is recognized early, it is normally treated with high-dose steroids or antibody therapy. Patients are normally asymptomatic although if severe, rejection can be accompanied by a flulike illness consisting of pyrexia and graft tenderness. It should always be considered in patients with deteriorating renal function and, as for ATN, the diagnosis is often very difficult, particularly in those with nonfunctioning grafts. Unfortunately, its presence has an adverse long-term prognostic indication.32 Although the B-mode appearances of acute rejection have been well-documented,33 these are now largely of a historical interest and should essentially no longer be observed, particularly in the postoperative transplant period, as they occur late, well after the onset of acute rejection, and are so arbitrary and inconsistent that they are of limited value. Such ultrasonic features include a reduction in corticomedullary differentiation, reduction in renal sinus echoes, increased and reduced renal parenchymal echoes, increased cortical reflectivity, and so forth. It is worthy of note, however, that observations of increased renal length34 and cross-sectional area35
Background
Introduction
Indications and Contraindications in Transplantation
Donor Supply
Histocompatibility Testing
Preoperative Management
Surgery
Immunosuppression
Imaging the Transplant Kidney
Early Complications
Acute Tubular Necrosis
Acute Rejection
Delayed Function
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree