Epidemiology
Aortoiliac occlusive disease (AIOD) refers to stenotic or occlusive disease of the infrarenal aorta and iliac arteries, and is a significant cause of peripheral arterial disease (PAD). Epidemiologic studies reporting on PAD include both AIOD and infrainguinal arterial disease, with most patients having multilevel disease. Although more than half of patients with PAD can have a component of AIOD, femoropopliteal and infrageniculate disease are more common causes of PAD. PAD is uncommon before age 50 but increases to around 20% over the age of 60 and over 40% over the age of 85. The prevalence of PAD is most commonly assessed with ankle-brachial pressure index (ABI) or by questionnaires. An ABI of <0.9 is used in clinical practice and epidemiologic studies to diagnose PAD, identifying both symptomatic and asymptomatic patients. The prevalence of asymptomatic PAD is reported as up to 11% and is higher in women than men. The prevalence of PAD is higher in low- and middle-income countries compared with high-income countries and varies by ethnicity, with higher rates reported in African Americans. Risk factors for PAD are well established (Fig. 13.1) . Patients with AIOD are more likely to be smokers and have a 2.5–3.5-fold risk of mortality and cardiovascular events compared with those with distal PAD, whereas patients with diabetes mellitus are more likely to have tibial disease as the cause of PAD. Global smoking trends show a significant decrease in the prevalence of tobacco use in high-income countries but increasing use of tobacco in low- and middle-income countries. At the same time there has been a doubling of the prevalence of diabetes mellitus between 1980 and 2014. This is likely to impact the future disease localization in PAD with increasing tibial disease and stable or declining AIOD, although temporal trends can lag several decades behind smoking trends.

Anatomic Classification of Aiod
The Trans-Atlantic Inter-Society Consensus (TASC) document classifies aortoiliac disease by angiographic appearance and pattern. It provides recommendations regarding percutaneous versus surgical revascularization based on severity of anatomic obstruction, describing anatomic lesions from a simple focal stenosis (TASC A) through to complex multifocal stenotic and occlusive disease (TASC D). Initially published in 2000, the guidelines were updated in 2007 as TASC II. A further update in 2015 expanded the TASC classification to include below-the-knee arteries, but due to a lack of contemporary randomized controlled trials no new guidelines were issued regarding revascularization of AIOD. It was recognized there had been significant change in clinical practice secondary to a rapid evolution in technology and increasing technical skill of endovascular specialists. Consensus was not achieved on the concept of recommending an “endovascular-first” approach for more complex TASC C and D lesions, and the update reflected a pragmatic approach to clinical practice, advising that the choice of revascularization method should be based on each vascular center’s experience and competence in treating complex AIOD. For complicated (TASC D) cases the update advised that primary surgical or hybrid endovascular and open strategies may be more appropriate in medically fit patients because the durability of an open approach over time may be superior to an endovascular procedure.
Clinical Evaluation
A directed history and physical examination should be performed to determine the presence and severity of PAD, bearing in mind that patients with hemodynamically significant aortoiliac disease are often asymptomatic, with only one-third presenting with intermittent claudication (IC). Clinical classification of systems for grading PAD are used widely in research and can also provide objective criteria for assessing the baseline disease state, disease progression, and treatment decisions for individual patients. The Fontaine Classification and Rutherford Classification are most widely used, and the Rutherford Classification includes clinical criteria with noninvasive objective criteria to assess the grade of PAD ( Table 13.1 ).
| Study Name | First Author, Year ∗ | No. of Subjects | Country | Population | Study Design | PAD End Point |
|---|---|---|---|---|---|---|
| Index studies | ||||||
| Cardiovascular Health Study | Newman, 1993 28 | 5084 | United States | Aged 65+ | Cross-sectional | ABI<0.9 |
| Framingham Study | Murabito, 1997 32 | 5209 | United States | Cross-sectional | IC | |
| Rotterdam Study | Meijer, 2000 33 | 6450 | Netherlands | Cross-sectional | ABI<0.9 (<0.7 also studied) | |
| Framingham Offspring Study | Murabito, 2002 26 | 3313 | United States | Longitudinal | ABI<0.9 | |
| Multiethnic Study of Atherosclerosis | Allison, 2006 34 | 6653 | United States | Cross-sectional | ABI<0.9 | |
| Other large studies | ||||||
| Quebec Cardiovascular Study | Dagenais,1991 35 | 4570 | Canada | Men only | Longitudinal | IC |
| Edinburgh Artery Study | Fowkes, 1992 36 | 1592 | Scotland | Cross-sectional | ABI and reactive hyperemia | |
| Israeli Ischemic Heart Disease | Bowlin, 1994 22 | 10059 | Israel | Middle-aged men | Longitudinal | IC projected |
| Reykjavik Study | Ingolfsson, 1994 21 | 9141 | Iceland | Men only | Longitudinal | IC |
| Honolulu Heart Program | Curb, 1996 37 | 3450 | United States | Japanese American men | Cross-sectional and longitudinal | ABI<0.9 |
| Limburg PAOD Study | Hooi, 2001 25 | 2327 | Netherlands | Longitudinal | ABI<0.95 | |
| Women’s Health and Aging Study | McDermott, 2000 38 | 930 | United States | Women aged 65+ | Longitudinal | ABI<0.9 |
| Physicians’ Health Study | Ridker, 2001 39 | 14916 | United States | Male physicians | Nested case-control | IC or PAD surgery |
| San Diego Population Study | Criqui, 2005 40 | 2343 | United States | Multiethnic | Cross-sectional | ABI<0.9, abnormal waveform, PAD revascularization |
| National Health and Nutrition Examination Survey | Pande, 2011 41 | 7458 | United States | American men | Longitudinal | ABI<0.9 |
| and women aged 40+ | ||||||
| Health Professionals Follow-Up Study | Joosten, 201 2 42 | 51529 | United States | Male health professionals | Longitudinal | Clinical PAD † |
∗ Where multiple articles were published, this refers to the article most frequently referenced herein.
† lncludes limb amputation or revascularization, angiography with vascular obstruction >50%, ABI<0.90, or physician-diagnosed PAD.
Clinical examination showing reduced or absent femoral pulses can indicate AIOD as the main territory contributing to PAD. Other vascular systems should be examined, including for carotid artery bruit or abdominal aortic aneurysm. Patients with AIOD can present with IC, most often affecting the calf muscles, with thigh or buttock claudication suggestive of more extensive AIOD. Symptoms of buttock claudication that occur in association with erectile dysfunction in patients with absent femoral pulses is recognized as Leriche syndrome. This syndrome occurs when either preocclusive stenosis or complete occlusion of the infrarenal aorta is present as a result of severe aortic atherosclerosis.
After a patient is identified with symptoms consistent with PAD it is important to rule out other potential etiologies that can mimic PAD symptoms, especially neurogenic claudication. Measurement of the ABI is the most widely used initial investigation for establishing a diagnosis of PAD. An ABI of <0.90 has been demonstrated to have high sensitivity and specificity for the identification of PAD.
Imaging Evaluation
Duplex Arterial Mapping
Duplex arterial mapping is still widely used in vascular laboratories for initial imaging of AIOD. For most patients it can provide a safe and inexpensive means of assessing the location and extent of hemodynamically significant stenosis in the aortoiliac territory with high sensitivity and specificity compared with computed tomographic (CT) angiography (CTA) and conventional catheter angiography. The presence of significant arterial calcification, overlying bowel gas, and large body habitus can exclude some patients from assessment with ultrasound. The need for highly trained vascular technologists can also limit access to duplex arterial mapping.
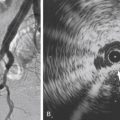
Targeted Doppler ultrasound can also be used to clarify findings on magnetic resonance and CTA studies when there is uncertainty that the degree of stenotic disease is at a threshold for intervention ( Fig. 13.2 ).

CTA for AIOD
Multidetector CT has rapidly evolved from 4-detector row systems in 1998 to 256-slice and 320-detector row CT systems with smaller detector element size and faster gantry rotation speeds. This allows a larger volume of coverage per gantry rotation, permitting rapid acquisitions of vascular anatomy over an extensive area. CTA of aortoiliac arteries are usually acquired as part of CTA of the thoracoabdominal aorta or as part of a peripheral CTA covering from the abdomen to the toes in a single acquisition. The increase in spatial resolution, with submillimeter isotropic acquisition, has seen an equally rapid development in image postprocessing and reconstruction from the acquired CTA three-dimensional (3D) dataset. Dedicated software can reconstruct the 3D data in any anatomic orientation with multiplanar reformatted, maximum intensity projection, and volume-rendered algorithms.
The improved spatial resolution and typical vessel size in the aortoiliac segment mean CTA is less affected by calcification than in the infrainguinal territory. The sensitivity of CTA for stenotic and occlusive disease may be higher than conventional angiography in AIOD, making it the diagnostic imaging of choice for many centers planning intervention of aortoiliac occlusive disease. Identification of extensive calcification in AIOD is important in planning intervention because it can be a predictor of complications, including rupture.
Magnetic Resonance Angiography for AIOD
Magnetic resonance angiography (MRA) is a noninvasive technique that does not employ ionizing radiation or iodinated contrast material and has benefited from significant developments in technology since it was first reported in 1985. MRA of peripheral vascular anatomy now reports high sensitivities and specificities for the detection of angiographically proven arterial stenotic and occlusive disease. Contrast-enhanced MRA has similar accuracy to CTA for classifying TASC lesions in AIOD. Future MRA sequences may hold promise for demonstrating arterial calcification that could mitigate the previous limitation of poor visualization of calcium when planning intervention in AIOD. Time-resolved contrast-enhanced MRA gives a dynamic assessment of arterial flow, but this is more frequently used for imaging infrageniculate PAD. MRA of the pelvis is still limited by susceptibility artifacts in patients with metallic hip implants.
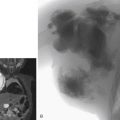
Non–contrast-enhanced MRA avoids the potential risks of nephrogenic systemic fibrosis and contrast-induced nephropathy, as well as ionizing radiation. Recent developments in non-contrast MRA sequences have overcome the previous limitations resulting from long acquisition time, flow and movement artifacts, and saturation effects as occurred with time-of-flight sequences. Quiescent interval single-shot MRA (QISS) appears best placed for imaging the lower extremities because the technique allows for large anatomic coverage in short imaging times. Although overall image quality is lower than CTA, Wu et al. reported high sensitivity (>93%) and specificity (>96%) for assessment of stenoses. They suggested this may be due to currently better spatial and temporal resolution for CTA (1 mm × 1 mm ×0.625 mm) than QISS MRA (1 mm ×1 mm ×3 mm). However QISS had significantly higher sensitivity for stenoses in heavily calcified vessels compared with CTA, which may be more relevant in AIOD ( Fig. 13.3 ).

Diagnostic Arteriography
Only a very small cohort of patients with AIOD may be considered appropriate for diagnostic catheter angiography. Almost all patients are able to have noninvasive imaging of the aortoiliac arteries by either CTA, MRA, or Doppler ultrasound, allowing patients to be planned for direct intervention for AIOD at the time of angiography. Patients with end-stage renal failure may not be suitable for intravenous gadolinium or iodinated contrast, but stenotic and occlusive disease can be mapped with Doppler ultrasound and unenhanced CT may have a role in demonstrating aortoiliac calcification before proceeding to intervention. CO 2 angiography can be performed with no recognized risk of causing renal impairment. It can be performed easily and safely, with minimal risk of air contamination, by a number of automated systems (Angiodroid SRL, San Lazzaro di Savena, Italy; CO2mmander CO 2 Injector, AngioAdvancements, Ft. Meyers, FL; INSPECT 3005R and 2005R, Malek Medical GmbH, Wismar, Germany). CO 2 angiography requires familiarity with the technical details of injection, and specific risks including vapor lock that can lead to nonocclusive mesenteric ischemia. Diagnostic catheter angiography can be performed via common femoral or radial artery approach, the latter allowing for rapid recovery and mobilization. Advancing developments in image fusion allow noninvasive diagnostic imaging to be fused with real-time fluoroscopic acquisition to help guide interventional procedures. This can reduce radiation exposure and contrast dose and will likely take a greater role in guiding interventional procedures.
Management of Atheromatous Aiod
Conservative
Cilostazol is a reversible selective inhibitor of phosphodiesterase 3 with antiplatelet, antithrombotic, and vasodilatory effects. It was approved by the US Food and Drug Administration in 1999 with a class I indication for intermittent claudication. It can significantly increase maximum walking distance and pain-free walking distance when compared with either pentoxifylline or placebo. It carries a black-box warning due to concerns about increased cardiovascular mortality in patients with congestive heart failure and use of phosphodiesterase 3 inhibitors. An analysis of the cilostazol safety database and a retrospective review of patient clinical records has not demonstrated an association between cilostazol use and major adverse cardiovascular event or stroke. However, the CASTLE study into the long-term effects of cilostazol reported adherence was poor: more than 68% of participants discontinued therapy by 36 months, and the most common adverse events reported include headache, diarrhea, and edema. However, the dropout rate did not differ significantly from patients in the placebo group (64%) and, although not suggested by the authors, could indicate challenges with compliance for medical therapy in patients with PAD.
Patients with IC secondary to AIOD can be managed with supervised exercise. The CLEVER (Claudication: Exercise vs. Endoluminal Revascularization) study looked at patients with >50% stenotic disease of the aorta or iliac arteries causing moderate to severe claudication. The study divided patients into three groups, randomly assigned to receive optimal medical care (OMC), OMC plus supervised exercise, or OMC plus stent revascularization. It showed that patients gained the greatest improvement in peak walking time with a supervised exercise program compared with either OMC alone or OMC with stent revascularization. Importantly, it also demonstrated the improvement in peak walking time was durable to 18 months, with an improvement in quality of life and was more cost-effective than stent revascularization. A limitation of the study noted by the authors was that only 11% of the eligible screened population were enrolled in the study, which may introduce bias relative to real-world practice. A Cochrane review of 21 studies, with a total of 1400 participants, concluded that supervised exercise programs improve maximal treadmill walking distance or time when compared with home‐based exercise therapy or when patients are given advice to increase physical activity levels by walking. This suggests that to maintain benefit from conservative management of AIOD with exercise, patients require ongoing support and supervision. An analysis of the cost-effectiveness of supervised exercise for AIOD suggested this would compare with well-established cardiac rehabilitation programs; however, it is not funded or widely available in many healthcare systems.
Combined Endovascular and Surgical or Hybrid Procedures
Combined endovascular and surgical or hybrid procedures for AIOD are most relevant for patients with significant common femoral artery stenotic or occlusive disease associated with iliac or aortic disease. Conventional surgery for extensive occlusive disease of the iliofemoral segment is open surgical reconstruction with aortobifemoral or iliofemoral bypass. Hybrid procedures are usually a combination of common femoral endarterectomy and endovascular treatment of aortoiliac diseased segments with self-expanding and balloon-expandable stents, both uncovered and covered. Most reports comparing open surgical and hybrid procedures for AIOD are single-center retrospective studies that demonstrate high levels of initial technical success of >90% for both open surgical reconstruction and hybrid procedures. Open surgical reconstruction for AIOD has higher primary patency rates of 80%–90% that are maintained out to 5 years, compared with hybrid revascularization with primary patency rates of 60%–80%. The higher patency rates with open surgery come with an up-front cost of longer hospital stays and higher morbidity and mortality. Significant between-study heterogeneity makes it difficult to document consistent differences; however, combined morbidity and mortality is estimated at 14.5%–20% for open surgery versus 8.5%–13% for endovascular approaches. Reduced procedural complications and development of endovascular techniques have significantly impacted the therapeutic approach to revascularization for patients with TASC C and D lesions with a shift from open surgery to endovascular intervention since the early 2000s. Recent studies in the “endovascular-first” era report outcomes for aortoiliac TASC C and D lesions have similar primary and 5-year patency for open surgery versus hybrid revascularization in low-risk patients. However, there remains a paucity of randomized controlled studies, as highlighted in the TASC II update, and the potential higher patency rates for open surgery compared with hybrid procedures for TASC D lesions is important to consider in planning individual patient treatment.
Open Surgical Procedures
Aortoiliac revascularization for TASC A and B lesions are now universally treated by an endovascular-first approach. Open surgical bypass is considered the gold standard for TASC C and D lesions, based on primary and long-term patency. However, as a result of the increasing use of endovascular treatment for TASC C and D lesions, the characteristics of patients treated with open surgery have changed over the past 30 years, as reported by Sharma et al. Medicare claims data from 1996 to 2010 indicate that the predominant therapeutic approach to revascularization for PAD shifted from open surgery to endovascular intervention around the year 2000. Since the turn of the new millennium, women and patients with comorbidities including hypertension, renal failure requiring dialysis, and smokers are less likely to be treated by surgery. Findings reported by Dorigo et al. support similar changes in treatment of TASC C and D lesions in a European teaching hospital setting. Patients treated with surgery are more likely to have had prior aortoiliac endovascular intervention.
Directly comparing outcomes between surgical and endovascular intervention for AIOD is challenging. The one randomized controlled trial comparing percutaneous balloon angioplasty and open surgical therapy for aortoiliac disease showed no statistically significant difference in outcome; however, patients were treated before 1987 and do not reflect current practice.
More recent studies are composed of single-center retrospective series for treatment of TASC B/C and D disease, with variation in practice and patient cohorts reflected in the differing outcomes for patency and complication rates.
Primary patency rates for aortobifemoral surgical bypass are reported as between 81% and 95% at 3–5 years compared with 65%–83% for endovascular management of AIOD, with secondary patency rates reported at 90%–97% for open surgical versus 82%–95% for endovascular repair. When comparing data for open versus endovascular treatment it is important to remember that primary patency in the surgical literature typically refer to the graft being “patent or not,” whereas for endovascular literature primary patency typically refers to absence of binary restenosis greater than 50%. This can lead to apparent inferiority in reported outcomes in endovascular literature for primary patency, and therefore secondary patency may be a better comparator to open surgery.
Complication rates are not universally reported in comparative studies of open surgical repair versus endovascular treatment for AIOD. Some studies report no significant difference in complication rates, although surgery resulted in significantly longer intensive care and total hospital stay. Dorigo et al. reported significantly higher cumulative postoperative complication rates for open surgical repair of 20.5% versus 7% for those undergoing endovascular repair. The trend toward treating TASC C and D lesions in AIOD with an endovascular-first approach may in part reflect this apparent difference in complication rates.
Endovascular Management
Endovascular revascularization of AIOD is ideally performed in a modern interventional radiology suite with access to high-quality fluoroscopy with software applications allowing for detailed image manipulation, multimodality integration, and cone-beam CT. Procedures can also performed in standard operating rooms with mobile fluoroscopy units but the limitations of this should be assessed when performing more complex aortoiliac reconstruction. Combined open surgical and endovascular procedures are optimally performed in a dedicated hybrid operating suite that combines operating room-grade airflow sterility and high-quality IR imaging. Additional important considerations include radiation protection, a contrast injector, and in-room ultrasound. Continuous patient monitoring is essential and requires cooperation among the patient, nurse, technologist, and interventionalist. A cardiac monitor, pulse oximeter, and automated sphygmomanometer are mandatory. Emergent patient resuscitation equipment including a code cart should be readily available, and all providers should be Advanced Cardiac Life Support (ACLS) certified. Working familiarity with conscious sedation medications including reversal agents is required. A “rupture box” with large-diameter occlusion balloons and covered stents was historically considered important to have available, although many centers now have these items available as standard inventory that can be quickly and easily accessed, obviating the need for a dedicated rupture box.
Preprocedure Patient Evaluation
Patients should be evaluated by the interventionalist before any procedure, ideally in a dedicated IR out-patient clinic. This allows time for a directed history and physical examination to determine the presence and severity of PAD, as outline previously. Complete assessment of the vascular system should include checking for a carotid artery bruit and abdominal aortic aneurysm. Previous test results should be reviewed, and where available pulse volume recording and segmental pressures obtained ( Fig. 13.1 ). Prior imaging with CTA or MRA and records of prior interventions and surgeries should be reviewed. Pertinent laboratory values should include platelet count, prothrombin time, serum creatinine, and glomerular filtration rate . Presence of severe renal insufficiency may preclude use of iodinated contrast agents and require consideration of CO 2 angiography or alternative agents such as gadolinium. A history of allergic reaction may require premedication with antihistamine and steroid or alternatively avoidance of contrast completely, if risk of anaphylaxis is suspected (e.g., previous laryngeal edema, bronchospasm, or cardiopulmonary collapse).
A clear-liquid diet is recommended for at least 6 hours before the procedure. On the morning of the procedure, conscious sedation evaluation should be performed, and peripheral intravenous access is required.
Indications
The two main indications for considering endovascular management of peripheral vascular disease are IC and critical limb ischemia (CLI). The decision to offer endovascular treatment to patients with IC depends on both patient and anatomic factors. Typically patients with moderate to severe claudication or CLI are considered for treatment. Often in the IC patient cohort treatment of the aortoiliac disease is sufficient for symptom relief even when concomitant femoropopliteal disease is present. Patients with CLI do not typically present with isolated aortoiliac disease, but rather multilevel disease. As previously described, with the increasing prevalence of diabetes mellitus in many populations these patients often present with tibial-dominant disease. Treatment of the aortoiliac disease in isolation is often insufficient for managing CLI, but is necessary to ensure good inflow to the lower treated segments.
Contraindications
Contraindications to arteriography in general also apply to aortoiliac interventions. Relative contraindications to elective percutaneous revascularization include patients with uncorrectable coagulopathy, inability to lie supine, severe non–dialysis-dependent renal insufficiency, or current systemic infections. For most patients these factors can be corrected or mitigated to allow endovascular management to proceed.
No absolute contraindications exist for endovascular treatment of aortoiliac disease per se, although some anatomic pathologies would rule out endovascular reconstruction, such as juxtarenal aortic occlusion, circumferential heavy calcification, and hypoplastic aortic syndrome. In some patients with infective AOID, an endovascular approach can be used to temporize or palliate until definitive open surgery can be performed.
Intervention
Common femoral artery puncture is the most frequent access for aortoiliac procedures. Historically femoral artery access was based on anatomic landmarks using the midfemoral head and palpation of the femoral pulse. Fluoroscopically guided puncture has been utilized in cases of absent or poor femoral pulses, or in the setting of obesity. Ultrasound-guided puncture is now the gold standard, allowing for accurate vessel access in the appropriate region as well as assessment of the common femoral artery for use of closure devices. Radial and brachial access are increasingly used, although radial access is limited by sheath size for larger-diameter devices. A recent meta-analysis (Meertens et al.) suggests transradial approach comparable to transfemoral approach in terms of success rates but with lower complication rates, although the data set was heterogenous. Axillary artery access may also be used in more complex aortoiliac reconstructions requiring renal protection or stenting (such as covered endovascular revascularization of the aortic bifurcation [CERAB]).
A sterile table should contain heparinized flush saline, hand-injectable contrast media, room for catheters and wires, lidocaine for dermal anesthesia, and a sharps container. The diagnostic portion of the examination can be performed using a multipurpose flush catheter .
Endovascular Equipment
- •
Vascular sheath
- •
Directional catheter
- •
Hydrophilic guidewire
- •
Exchange wires
- •
Angioplasty balloon
- •
Self-expanding stent
- •
Balloon-expandable stent (mounted or unmounted)
- •
Covered stent
- •
Reentry catheter
- •
Hemodynamic pressure monitor
- •
Closure devices
There is an extensive inventory of interventional radiology equipment used in the treatment of AIOD that will differ by geographic region, country, or individual units because of availability or cost. We outline the general principles to consider when selecting equipment specifically for AIOD.
For choosing the appropriate vascular sheaths required for a procedure, a detailed understanding of device–sheath compatibility is important when planning cases. Initial access with a standard 5F or 6F short sheath to allow for crossing of stenotic or occlusive disease is typical. If required, sheath size can then be increased to allow for treatment device passage. Iliac vessel diameter typically ranges between 6 and 12 mm, with most standard angioplasty balloons and self-expanding stents for these diameters being delivered through 5F or 6F sheaths. For placement of balloon-expandable stents, a vascular sheath that can deliver the stent to the target segment without the risk of stent dislodgement from the delivery balloon catheter is required. This requires larger-diameter and longer sheaths that are resistant to kinking in tortuous iliac anatomy, and 6–12F sheaths in lengths of 25–65 cm should be available, as per the device manufacturers’ Instructions for Use. If iliac calcification or vessel size on preprocedure imaging raises concern for a risk of rupture, then a sheath capable of delivering and supporting a large-diameter occlusion balloon, such as a CODA balloon (Cook Medical, Bloomington, IN) or similar occlusion balloon, should be available.
Many varieties of catheters and guidewires are available for treatment of AIOD. Simple stenosis may only require a straight tapered spring-coil guidewire. A combination of hydrophilic guidewires (e.g., Glidewire [Terumo Medical Corp., Somerset, NJ]) and directional catheters with short multipurpose angled tips can be used for complex stenosis and occlusions. Exchange-length guidewires with greater stiffness (e.g., Rosen or Amplatz [Cook Medical]) are needed to pass subsequent sheaths, balloons, and stents. Although larger-diameter angioplasty balloons up to 15 mm may be necessary for distal aortic purposes, many patients will experience symptomatic relief with 10- or 12-mm-diameter stents, even in the aorta. Dilation to larger diameters increases the risk of aortic rupture. For iliac arteries, balloon diameters between 6 and 10 mm suffice, usually 7–9 mm in the common iliac arteries and 6–8 mm in the external iliac arteries. Inflation devices with an atmospheric pressure gauge allow for controlled calibrated inflation of semicompliant balloons to the appropriate diameter as per the Instructions for Use should also be available.
Vascular Stents in AIOD
Balloon angioplasty is rarely performed alone when treating AIOD because the primary patency is inferior to stenting, primarily due to residual stenosis secondary to elastic recoil and arterial dissection. Bare-metal stents are generally classified as balloon expandable (BE) and self-expanding (SE). The advantages of BE stents include accurate positioning and high radial resistive force. SE stents provide better conformability and an ability to cope with vessel deformity with motion. There is surprisingly little objective evidence comparing BE and SE stents in AIOD. The ICE trial was the first randomized controlled trial comparing SE and BE stents in common and external iliac arteries Most (58.9%) patients were treated for common iliac artery (CIA) stenotic or occlusive disease. The trial reported better outcomes using SE stents in the iliac arteries compared with BE stents, with significantly lower binary restenosis (6.1% vs. 14.9%), 12-month primary patency (94.5% vs. 87.0%) and 12-month freedom from target lesion revascularization (TLR) (3.0% vs. 6.9%). The authors speculated that the lower radial force and higher elasticity of SE stents may help prevent circumferential stress, therefore reducing neointimal proliferation, while also preserving arterial distensibility. Although only suggested by post hoc analysis, the benefit of SE appeared more doubtful in heavily calcified lesions. The trial did not report separately on treatment of CIA ostial lesions or the use of “kissing-stent” iliac reconstruction.
In the BRAVISSIMO trial comparing the Abbott Vascular BE (Omnilink Elite) and SE (Absolute Pro) stents, no significant difference was seen in 12-month primary patency between the stent platforms. Indeed, the best results were obtained when a combination of stents was used. Similar results were seen in the Medtronic-sponsored Durability and Visibility iliac artery trials. A common stent algorithm in AIOD is to use BE stents at the aortic bifurcation and common iliac arteries where high radial resistive force is important because disease is often resistant and calcified. SE stents are more commonly used in the external iliac arteries because this vessel is distorted by hip movement. SE stents tend to be more trackable and deliverable, which is relevant when treating contralateral iliac artery disease across the aortic bifurcation.
In recent years, there has been a shift to treating complex AIOD with covered stents, particularly BE covered endoprostheses. These devices may have a number of advantages over uncovered stents, including a reduced risk of arterial perforation or rupture, prevention of in-stent neointimal hyperplasia, and plaque protrusion through the stent. The COBEST trial comparing uncovered stents to the Gettinge Atrium V12 (iCast) BE covered stent found superior early patency for the covered stent in more complex TASC C and D lesions. This advantage was sustained to 5 years. Excellent results favoring other BE covered stents have been reported, including the Gore VBX and Bard Lifestream devices. SE covered stents have been used less frequently in AIOD, although the Gore Viabahn has been successfully used to recanalize iliac artery chronic occlusions, particularly the external iliac artery.
Procedural Antiplatelet and Anticoagulant Medication
Patients with AIOD are frequently on antiplatelet or anticoagulant medications because antiplatelet agents have been shown to reduce cardiovascular events and death in patients with intermittent claudication. Antiplatelet agents should be continued periprocedurally in most patients except where the risk of bleeding is considered high. Although there are no large prospective randomized studies looking at complication rates in patients taking periprocedural antiplatelet agents, a clear increase in the rate of myocardial adverse events has been shown in patients stopping antiplatelet agents before open surgery. Because of the paucity of published data, consensus guidelines, such as those regularly updated by the Society of Interventional Radiology, provide an evidence-based approach to periprocedural management of antiplatelet and anticoagulant medications.
Warfarin compounds and metformin should be withheld when appropriate. An assessment of cardiovascular risk and periprocedural bleeding risk should be made as a shared decision with the medical team managing the patient and a consensus decision made regarding continuing or stopping oral anticoagulants, and whether bridging therapy with low-molecular-weight heparin should be considered.
Intraprocedural use of unfractionated heparin is the most commonly used antithrombotic agent in percutaneous peripheral vascular interventions. Kasapis et al. studied unfractionated heparin use in peripheral vascular interventions showing that the use of weight-based heparin dosing, with an initial bolus dose up to 60 U/kg, and a target activated clotting time (ACT) <250 seconds may minimize the bleeding risk without compromising procedural success or increasing thromboembolic complications.
Case Management
AIOD often involves multilevel disease from the infrarenal aorta to the distal external iliac and common femoral arteries, although isolated segmental disease also occurs. The principles for treatment of aortoiliac arterial segments differ and are ideally discussed as discrete territories, which can then be applied when performing multilevel endovascular management.
Infrarenal Aortic Revascularization
Focal stenoses of the infrarenal aorta are relatively uncommon but may be treated with stenting using either BE or SE stents. Chronic occlusion of the infrarenal aorta is more common and often involves bilateral common iliac artery occlusion. Patients frequently present with Leriche syndrome—buttock and thigh claudication often association with erectile dysfunction in males. Endovascular management of TASC D aortoiliac occlusion is becoming the first-line approach achieving excellent patency while avoiding the significant morbidity and mortality of open surgical repair. Endovascular approaches include “kissing” double-barrel stents, CERAB, and bifurcated endografts. The use of kissing covered stents has the advantage of procedural simplicity and can normally be performed via retrograde groin approaches. However, there are several potential disadvantages, including long, small-caliber flow lumens, possibly with reduced patency. Raising the aortic neobifurcation may also have hemodynamic consequences. In addition, bilateral subintimal recanalization may result in endografts being deployed in different subintimal spaces, which may be problematic. There is limited evidence supporting the double-barrel approach but single-center case series have reported encouraging results.
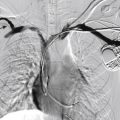
The CERAB technique will be described in detail in the section on aortic bifurcation revascularization. However, this technique can be extended to treat TASC D aortoiliac occlusions. When performing these procedures, upper limb access is often extremely helpful. Not only does the antegrade passage of a guidewire often cross aortic occlusions more successfully than a retrograde approach but the upper limb access also allows deployment of parallel grafts to maintain patency of renal or mesenteric branches ( Fig. 13.4 ). Conventional bifurcated endografts, primarily designed for aortoiliac aneurysm disease, have also been used in extensive aortoiliac occlusive cases. A number of endografts have been used in this setting but one of the most widely used is the Endologix AFX, with a unibody design that sits on the aortic bifurcation. This reduces the risk of limb compression, which is prevalent in the constrained anatomy of aortoiliac occlusive disease. However, the AFX, like all current endografts designed for aneurysm disease, lacks the radial resistive force that is often required in AIOD, and relining with BE stents is often required.