Acknowledgment
The authors express their gratitude to Dr. Mahmood Razavi for his insight and support in writing this chapter.
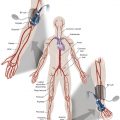
Femoropopliteal occlusive disease is present in a significant proportion of older patients, with about 20% of men and 17% of women older than 55 living with ankle-brachial indices (ABI) under 0.9. A smaller percentage (3%–7%) present with symptoms of intermittent claudication (IC) between the ages of 60 and 70. After the age of 70, however, 25% of patients with IC clinically deteriorate, with 1%–3.3% progressing to critical limb ischemia and eventual amputation. Although only some patients experience worsening claudication or gangrene, many more adjust to this reduction in circulation by becoming progressively more sedentary. As such, the femoropopliteal arteries deserve respect as critical determinants in the health and mobility of our older citizens. To be sure, significant lower extremity occlusive arterial disease has ominous associations with generalized vascular disease, and the estimated 5-, 10-, and 15-year morbidity and mortality of these patients is 30%, 50%, and 70%, respectively, with 70% to 80% of deaths related to cardiovascular events (myocardial infarction, cerebrovascular accidents, and ruptured aneurysms). These figures are 2.5 times higher than that of nonclaudicants.
The anatomy and physiology of the femoropopliteal artery is unique and poses specific challenges for both percutaneous and surgical treatments. The femoral artery is the longest artery in our body, and has the fewest branches. It has thick muscular walls so that it can tolerate the variety of longitudinal and lateral compressive forces delivered to it by virtue of its fixation at the highly mobile hip and knee joints. In this milieu, catheter-based intervention often falters because this powerful artery scars, proliferates, and thromboses under the injuries inflicted by angioplasty, and it fractures and deforms metallic stents placed within its lumen. No wonder that multiple devices have tried and failed to tame occlusive disease in the femoropopliteal arteries.
In this chapter, the discussion of femoropopliteal occlusive disease begins with its clinical and noninvasive diagnosis. Then established medical and surgical therapies are described. Finally, the chapter focuses on endovascular methods, including the multitude of past failures and their evolution to more modern devices and treatments available today and in the near future.
With more than 5000 articles pertaining to femoral artery stents available in the recent medical literature, the importance of critical manuscript review in this subject area cannot be overstated. Therefore, the reader must consider important comparative data when evaluating the various treatments for femoropopliteal arterial disease, including, but not limited to, the status of the runoff and inflow vessels, the length and degree of calcification of lesions treated, clinical versus imaging follow-up, and the presence of concomitant disease states such as diabetes, continued smoking, or renal failure.
Lexicon and Nomenclature Updates
In early 2019, the three major global vascular surgical societies—the European Society for Vascular Surgery, the Society for Vascular Surgery (SVS), and the World Federation of Vascular Societies—came together and launched a joint initiative of global vascular guidelines. Under the initiative the peripheral arterial disease nomenclature was redefined. Previously used terms, such as “critical” and “severe” limb ischemia are very specific to certain defined hemodynamic thresholds and fail to encompass the complete spectrum of, and interrelatedness of, components other than ischemia that majorly contribute to limb amputations and long-term disability.
Peripheral arterial disease (PAD) involving the lower extremities is defined as the presence of disease-causing hemodynamic compromise resulting in a resting ABI of less than 0.9. The clinical presentation of PAD can range from no symptoms to gangrene and tissue loss. PAD is a strong marker for the presence of systemic atherosclerotic disease and is therefore associated with an increased risk of major cardiovascular events (5%–7% annually). The most common cause of occlusive disease in the infrainguinal vasculature, in particular in the femoropopliteal segment (FPS), is atherosclerosis; the major risk factors are presented in Table 14.1 .
| Risk Factor | Risk of Developing Peripheral Vascular Disease | Threshold Value | Means of Risk Reduction | Target |
|---|---|---|---|---|
| Race | 7.8% vs. 4.4% non-Hispanic blacks over whites | NA | NA | NA |
| Sex | 2 or 3:1 males over females | NA | NA | NA |
| Age | 2.5% age 40–59 y, increasing to almost 20% over age 70 y | NA | NA | NA |
| Smoking | OR 2.6 for men; 4.6 for women | Any smoking | Combination of physician encouragement, bupropion, and nicotine replacement therapy | Cessation of smoking has twofold increase in 5-year survival rate and threefold decreased risk of amputation compared with continued smoking |
| Hypertension | OR 2.2 for men; 2.8 for women | Over 140 mm Hg systolic and 90 mm Hg diastolic | Aggressive medical management | Below 140/90 mm Hg generally, but below 130/80 mm Hg in patients with diabetes or in renal failure |
| Diabetes | OR 6.1 for men; 3.6 for women | Persistently elevated glucose | Insulin if not controlled with antihyperglycemic agents | Hemoglobin A1c under 7% |
| Hyperlipidemia | OR 1.02 for each 1 mg/dL increase in serum LDL in men or women | Normal range | Diet before statins, niacin, or fibrates | Keep LDL below 100 mg/dL (2.59 mmol/L) |
a Other risk factors: hyperhomocysteinemia, hypothyroidism, increased inflammatory markers (C-reactive protein), increased blood viscosity, chronic renal failure.
Intermittent claudication (IC) is the most common symptom related to PAD. Typical symptoms consist of muscle fatigue, ache, or cramps induced by exertion and relieved by rest (Rutherford-Becker classification II). Involvement of calves, thighs, or buttocks indicates the level of anatomic disease.
Chronic limb-threatening ischemia (CLTI) is the most severe manifestation of PAD. Since 1982 critical limb ischemia has been defined as “ischemic rest pain” with an ankle pressure (AP) < 40 mm Hg, or tissue necrosis with an AP < 60 mm Hg, in patients without diabetes. Given the confounding effects of neuropathy and susceptibility to infections, patients with diabetes were specifically excluded. This definition, however, fails to encompass a large group of patients who were at risk for amputation from a broader range of ischemia. The global vascular guidelines, however, promote the use of the term chronic limb-threatening ischemia as a “clinical syndrome,” defined by the presence of PAD in combination with rest pain, gangrene, or a lower limb ulceration >2 weeks’ duration. Venous, traumatic, embolic, and nonatherosclerotic etiologies (such as vasculitides, collagen vascular disease, Buerger disease, neoplastic disease, dermatoses, and radiation arteritis) are excluded. Thus, the term CLTI is preferred.
In terms of classification schemes, several lower limb ischemia and wound/diabetic foot ulcer classification systems have been developed. For example, the Fontaine and Rutherford classifications have been popular among vascular surgeons and interventionalists, whereas orthopedists, podiatric surgeons, and diabetic foot specialists traditionally applied the Wagner and University of Texas classifications. The Fontaine classification is solely based on clinical staging, from Stage I (asymptomatic, incomplete vessel obstruction) to Stage IV (necrosis/gangrene of the limb). The Rutherford classification helps categorize both acute and chronic limb ischemia, utilizing both clinical and objective criteria (e.g., Doppler imaging, treadmill exercise testing) to guide treatment algorithms. The reader is encouraged to familiarize themselves with these classifications because their use is still popular among clinicians.
Each of these systems has its own strengths and limitations. The use of multiple classification systems has always hindered the development of optimal treatment algorithms both for diabetic and nondiabetic patients across the spectrum of PAD. Even though these previous treatment guidelines and classifications systems have suggested a range of hemodynamic thresholds for AP and toe pressure (TP) for defining limb-threatening ischemia, such thresholds lack a clear and reliable relationship to outcomes. As such, these definitions were ischemia-dominant models of limb threat due to atherosclerosis, with smoking being the predominant risk factor and excluded the defining role of the modern-day diabetes mellitus (DM) epidemic.
However, given that DM now makes up the majority of risk factors for CLTI, the concept of absolute perfusion is now considered in the context of neuropathy, wound characteristics, and infection. The SVS Lower Extremity Guidelines Committee created the SVS Lower Extremity Threatened Limb Classification System, which classifies amputation risk according to wound extent, degree of ischemia, and presence and severity of foot infection, known as WIfI (Wound, Ischemia, and foot Infection). The WIfI classification system appears to correlate strongly with important clinical outcomes. This in turn serves as an integral tool in clinical decision-making, for predicting which patients might fare better with open surgical bypass compared with endovascular therapy.
Diagnosis
As described earlier, diagnosis of CLTI requires objective documentation of atherosclerotic PAD in the clinical settings of ischemic rest pain or tissue loss (ulceration or gangrene). The key physical examination components in patients with PAD include palpation of extremity pulses and evaluation of the extremities for color, temperature, chronic hair loss, nail changes, and skin ulcerations. Those patients with a history and/or physical findings suggestive of PAD should proceed to more objective testing, including the ABI.
Ischemic rest pain is classically defined as affecting the forefoot, which is worsened with recumbency and relieved by dependency. It must persist for >2 weeks and be associated with one or more abnormal hemodynamic parameters. These parameters include an ABI < 0.4 (using pressure more proximal to the dorsalis pedis and posterior tibial arteries), absolute highest AP < 50 mm Hg, absolute TP < 30 mmHg, transcutaneous partial pressure of oxygen (TcPO 2 ) < 30 mm Hg, and flat or minimally pulsatile pulse volume recording waveforms (WIfI ischemia grade 3 equivalent). In patients with DM and end-stage renal disease, pressure measurements should be correlated with Doppler arterial waveforms. The AP and ABI in this patient population are frequently falsely elevated because of medial arterial calcinosis. Hence, in patients with DM or end-stage renal disease, toe waveforms and systolic pressures are preferred. Recent data also suggest that TP and TcPO 2 measurements are more accurate than AP and more predictive of 1-year amputation risk (TP < 30 mm Hg or TcPO 2 < 10 mm Hg). CLTI-related tissue loss includes gangrene of any part of the foot or nonhealing ulceration present for at least 2 weeks. Gangrene typically involves the digits or the heel and may progress to affect the more proximal forefoot. Objective evidence of significant PAD should be documented (e.g., WIfI classification). Of note, these diagnostic criteria exclude patients with purely neuropathic, traumatic, or venous ulcers lacking any ischemic component.
Imaging of Vascular Anatomy
Doppler velocity waveform analysis is also a useful tool that is frequently performed as part of a formal noninvasive vascular study. As PAD progresses in severity, triphasic arterial waveforms progressively become biphasic, then flat or monophasic as arterial flow diminishes.
Vascular imaging is performed in all patients with documented CLTI to determine the extent and severity of disease and to plan for revascularization. Digital subtraction angiography remains the gold standard. However, accurate diagnoses can be achieved with noninvasive modalities such as duplex ultrasound (DUS), computed tomographic angiography (CTA), and magnetic resonance angiography (MRA). Digital subtraction angiography is typically reserved for when endovascular treatment is contemplated.
DUS is usually the first-line imaging modality of choice and provides information on the anatomic location and extent of disease as well as information about flow volume and velocity. Even though it is a noninvasive modality and low cost, DUS is time-consuming and highly operator-dependent, and it does not produce a continuous lesion map or definitive information of collateral supply reserves.
CTA and MRA are highly sensitive and specific for arterial occlusive disease in the large and medium-sized vessels, but can be limited in patients with densely calcified arterial disease. Most practitioners reserve costly cross-sectional imaging for those patients with symptoms warranting intervention and segmental limb pressures indicative of potentially correctable arterial disease. A more detailed discussion of CTA and MRA is presented elsewhere in this book.
CO 2 angiography can be used as an alternative to iodinated contrast in patients with an allergy to contrast material or in individuals with severe chronic kidney disease. Nonetheless, the imaging performance becomes progressively degraded in the more distal infrapopliteal arteries.
Perfusion angiography is a fairly novel technique performed with use of a dedicated imaging suite and workstation. This not only provides time-resolved perfusion imaging of the foot to aid in the diagnosis and impact of revascularization techniques, but also quantifies the functional status of foot perfusion.
Management and Treatment
PAD—in particular, CLTI—includes a spectrum of systemic atherosclerosis, hence it is almost always accompanied by clinically significant cardiovascular disease, associated with exceedingly high mortality from stroke and myocardial infarction. The ultimate goal of treatment should not only be to salvage a functional limb but also to aggressively identify and treat all modifiable risk factors. These include hyperlipidemia, hypertension, diabetes, smoking, and sedentary lifestyle.
Antithrombotic Therapy
In light of all available data and trials since the advent of antiplatelet agents, it is indeed a well-established fact that antithrombotic therapy serves as the cornerstone of management of symptomatic PAD. A growing body of literature indicates that antithrombotic therapy not only reduces the risk of major adverse cardiovascular events (MACEs: death, myocardial infarction, or stroke) but also major adverse limb events (MALEs: major amputation, repeated revascularizations, and acute limb ischemia).
Many pharmacologic agents and combination regimens have been studied and approved by the US Food and Drug Administration. having shown evidence of clinical significance. Historically these have included aspirin and alternatives to aspirin, such as ticlopidine, dipyridamole, and clopidogrel. The most popular of these is the daily combination therapy of low-dose aspirin (81–325 mg) and clopidogrel (150 mg). However, most recent developments in the pharmaceutical industry and extensive trials and meta-analyses have brought to light some very novel agents and recommendations. For example, the most notable of these is the “Clopidogrel versus Aspirin in Patients at Risk for Ischemic Events” (CAPRIE) trial. , This study suggests that although 75 mg of clopidogrel per day as a single antithrombotic therapy has a similar safety profile to 325 mg of daily aspirin, it results in comparatively more significant decreases in MACEs compared with 325 mg of aspirin per day in patients with PAD. Finally, studies have found that in patients with PAD, clopidogrel monotherapy resulted in the best overall safety and efficacy profile in prevention of MACEs.
Vorapaxar (protease-activated receptor 1 antagonist), an emerging class of antithrombotic, has shown some promising results in the reduction of MACEs, ischemic strokes, and MALEs in PAD patients. However, it has been associated with higher incidence of intracranial hemorrhage. Similarly, combination therapy of low-dose rivaroxaban (an oral factor Xa inhibitor) with aspirin has shown significant reduction in MACEs and MALEs compared with aspirin alone. However, the combination renders a worse bleeding profile than monotherapy with these agents. Dual antiplatelet therapy or systemic anticoagulation with vitamin K antagonists has not been indicated as a monotherapy for PAD or CLTI.
Lipid-Lowering Therapy
Blood lipid lowering has a significant effect on cardiovascular events in PAD. Lipid lowering has been shown to be most effective in patients with a blood cholesterol concentration > 135 mg/dL (>3.5 mmol/L). In addition, like blood pressure and cholesterol, inflammatory biomarkers have been proven to be an independent predictor of vascular risk. Recent studies also demonstrated a significant reduction in major vascular events, including myocardial infarction, stroke, arterial revascularization, deep venous thrombosis or pulmonary embolism, and mortality. The greatest absolute risk reduction has been seen among patient cohorts with the highest levels of high-sensitivity C-reactive protein. The American College of Cardiology/American Heart Association guidelines dictate the use of moderate- to high-intensity statins for all individuals with established atherosclerotic cardiovascular disease including PAD. The statin agents may have beneficial effects beyond their lipid-lowering effect by reducing inflammation in patients with PAD. Evident decrease in cardiovascular events is noted in populations with established atherosclerosis treated with intensive statin therapy. The main limiting side effect of statin therapy has been muscle aches. In these instances, it is recommended to decrease the dose to the maximum tolerated dose with addition of a second nonstatin cholesterol-lowering drug. Newer lipid-lowering agents such as proprotein convertase subtilisin/kexin type 9 (PCSK9) have recently emerged as comparatively beneficial alternates with better side–effect profile and ease of administration with 6-monthly depot doses. These agents direct the degradation of low-density lipoprotein receptors in the liver and have become a drug target. Extensive cardiovascular outcomes research has also proved additional benefits of PCSK9 inhibition in combination with statin therapy in reducing MACEs and MALEs in the PAD population.
Management of Hypertension
Although maintaining a “healthy” blood pressure, systolic pressure < 140 mm Hg and diastolic pressure < 90 mm Hg (shown to be beneficial to reduce MACEs in PAD), is often advocated, a precise target blood pressure for patients with CLTI has not been defined. Furthermore, it is accepted that overcontrol of blood pressure may itself result in morbidity from excessive hypotension and worsen ischemia in PAD. No definitive first-line category of antihypertensive has been established. Calcium channel blockers, angiotensin-converting enzyme inhibitors, and diuretics are equally effective in reducing cardiovascular events if successful in bringing the blood pressure to target range. However, recent trials such as the Heart Outcomes Prevention Evaluation (HOPE) study suggest some increased amputation rate associated with angiotensin-converting enzyme inhibitor monotherapy in patients with CLTI in the absence of heart failure. Similarly, use of beta-blockers after primary vascular reconstruction results in comparatively decreased risk of major amputations, but these drugs have worse safety profiles in terms of myocardial infarction and stroke-related morbidity.
Of note, several vasodilatory agents have been studied for the relief of symptoms of IC. Only a few, however, have shown any evidence of clinical utility, and they do not provide the same level of symptom relief as a successful revascularization. The first Food and Drug Administration-approved agent for claudication was pentoxifylline, a methylxanthine derivative thought to increase red blood cell membrane deformability and decrease fibrinogen activity and platelet adhesion, with the overall effect of reduced blood viscosity. Multiple trials have failed to show considerable clinical benefit, so this drug is probably beneficial only to those who cannot tolerate cilostazol, a better claudication drug. Cilostazol is a phosphodiesterase 3 inhibitor with some antiplatelet and vasodilation activity. As such, it should not be prescribed to patients with congestive heart failure, owing to decreased survival compared with placebo in patients with class III–IV congestive heart failure . Cilostazol increases maximal and pain-free walking distances by half and two-thirds, respectively, after 3 to 6 months of therapy, but its side effects of diarrhea, headaches, and dizziness present a compliance issue, with up to 60% of patients stopping the drug within 3 years.
Management of Diabetes
The extent of vascular disease manifestations in PAD is directly proportional to the duration and severity of hyperglycemia. The goal for most DM-associated PAD cohorts is to maintain a glycosylated hemoglobin A1c level of <7% (8% in advanced vascular disease with limited life expectancy). Metformin monotherapy is hailed as the best and first-line oral hypoglycemic agent. For added control and therapy, any of the other class of oral hypoglycemic agent, including sulfonylurea, thiazolidinedione, dipeptidyl peptidase 4 inhibitor, or alpha-glucosidase inhibitors, are considered equally effective. However, metformin has proven associated higher risk of lactic acidosis in patients with poor renal function, with recommendations to withhold metformin for 48 hours after administration of iodinated contrast agents. A novel class of antiglycemic agents, sodium-glucose cotransporter-2 inhibitors, have emerged as comparatively protective for cardiovascular and renal complications in diabetics. Nonetheless, of these sodium-glucose cotransporter-2 agents, canagliflozin has notoriously established itself to have twofold increased associated risk for lower limb amputations in CLTI patients, prompting a black-box warning.
Lifestyle Modifications
The importance of lifestyle modifications, such as permanently stopping smoking, following a low-fat Mediterranean diet, getting exercise, and maintaining weight control cannot be overstated. The safety of electronic cigarettes in CLTI remains questionable and needs to be explored further. Improvement in plaque burden is well established with diet modifications and exercise both before and after vascularization.
Other critical elements of treatment include adequate pain control and treatment of infections if present. A graded or tiered approach to pain management is recommended, with a tradeoff between benefits and harms (e.g., constipation, drowsiness). Care of ulcerations will require specialized treatment and should be done in conjunction with specialists in foot and wound care. Primary major amputation may become necessary if anatomic or comorbid medical conditions do not allow for a successful revascularization. Frequently, minor amputations are done after a revascularization procedure for limb salvage.
Evidenced-Based Revascularization
The concept of evidence-based revascularization (EBR) remains the cornerstone of limb salvage in CLTI. The primary goals and endpoints of treatment are relief of pain, healing of wounds, and preservation of independent ambulatory functional limb status. The latest global vascular guidelines group consensus 2019 has proposed an evidenced-based, structured approach to the management of CLTI. This novel systemic paradigm is a fundamental departure from the previously described management algorithms used in management and classification of PAD. These consensus guidelines propose an integrated three-step approach for EBR based on the following factors:
- •
Patient risk estimation
- •
Limb staging (WIfI limb staging and ischemia grading)
- •
Anatomic pattern of disease (the Global Limb Anatomic Staging System [GLASS])
Patient risk estimation involves assessing patient candidacy for limb salvage by assessing periprocedural risk, life expectancy, comorbidities, and frailty. To date, multiple operative risk tools are available and currently used in various practices, which primarily take into consideration procedural morbidity and mortality, repeated revascularization, postprocedural outpatient care, and social care costs. These risk-assessment tools have largely been based on retrospective studies and do not incorporate the patient cohorts managed conservatively or treated with amputation or palliation. As such, no single tool or risk-assessment model is primarily preferred over the others and a detailed discussion and comparison is beyond the scope of this book. The reader is referred to follow their group or institute’s preferred assessment algorithms. However, a thorough estimation of perioperative revascularization risk assessment and shared clinical decision-making with the patient, family, and the proceduralist is critical to EBR. It must involve detailed discussion of the tradeoff between revascularization-associated mortality/morbidity with palliation or amputation, should the risks be critically high.
Limb staging , both clinical and functional, is fundamental in the procedural planning for EBR of CLTI patients. The previously presumed concordance of severity of ischemia and beneficial outcomes of revascularization in the prior management classification models are no longer considered exclusively correlative. The WIfI threatened limb classification system redefines the benefits of revascularization in CLTI in light of both severity of ischemia and degree of limb threat, particularly in terms of the degree of tissue loss and concomitant infectious processes. This is the only system that also serves as a decision-making tool to compare the quality of different revascularization strategies in CLTI. Clinically symptomatic patients with severe ischemia (WIfI ischemia grade 3) must undergo attempted revascularization. Similarly, in patients with advanced tissue loss or infection (WIfI limb stage 4), revascularization may also be of benefit in the presence of moderate ischemia (WIfI ischemia grades 1 and 2).
In contrast, patients with milder tissue loss or infection (WIfI limb stages 1–3) and mild to moderate ischemia (WIfI ischemia grades 1 and 2) are preferentially treated with infection control and podiatric wound care. These patients undergo 4–6 weeks of wound care, and if the wounds fail to improve, revascularization may be considered. Periodic restaging of the limb (WIfI limb stage) over the course of treatment is central for successive decision-making.
Anatomic pattern and staging of disease is the backbone of EBR and an integral consideration in the management of CLTI. The global vascular guidelines group consensus 2019 has introduced a new horizon approach to classifying the anatomic pattern of arterial disease. The prior and current anatomic classification systems fundamentally focused on severity of localized individual arterial lesions. These systems did not consider the multilevel nature, systemic complexity, and increasingly distal involvement of the CLTI disease patterns, as encountered in current clinical practices. Hence, they were not useful in comprehensively defining EBR strategies along the spectrum of management of CLTI. The new Global Limb Anatomic Staging System (GLASS) is based on defining a target arterial pathway (TAP) with the help of high-quality imaging, which is identified as the least diseased arterial channel providing runoff to the foot. The TAP considers multiple lesions in series and defines the CLTI disease process as a product function of each lesion traversed. This serves as a major departure from the previously described individual lesion-based anatomic classification schemes and focuses on integrating and developing management approaches for considered arterial segments in the whole limb. Of note, the new GLASS system mainly focuses on infrainguinal disease as a unit process and considers the common femoral artery and profunda femoris artery as inflow vessels. The superficial femoral artery (SFA) and the malleoli are considered as the proximal and distal anatomic landmarks, respectively, for defining the infrainguinal system. The degree of vessel calcification and inframalleolar disease are considered important modifiers and not integral defining factors for the final grading and staging of the TAP disease burden. The final composite infrainguinal anatomic GLASS stages (I–III) are synthesized by aggregating separately calculated grades for the femoropopliteal and infrapopliteal segments, in combination with limb-based patency (LBP) ( Table 14.2 ). Where LBP is defined as maintenance of the pulsatile in-line flow through the entire length of the TAP (from the SFA origin to the malleoli), the three GLASS stages are defined in reference to the likelihood of immediate technical failure and 1-year LBP ( Table 14.3 ).
| Infrainguinal GLASS Stage (I–III) | ||||||
|---|---|---|---|---|---|---|
| 4 | III | III | III | III | III | |
| 3 | II | II | II | III | III | |
| FP Grade | 2 | I | II | II | II | III |
| 1 | I | I | II | II | III | |
| 0 | NA | I | I | II | III | |
| IP Grade | 0 | 1 | 2 | 3 | 4 | |
| Estimated PVI Outcomes | |||
|---|---|---|---|
| Stage | Technical Failure | 1-Year LBP | Anatomic Pattern |
| I | <10% | >70% | Short- to intermediate-length FP disease and/or short-length IP disease; no or minimal popliteal disease |
| II | <20% | 50%–70% | Intermediate- to long-length FP disease; may include popliteal stenosis and/or short- to intermediate-length IP disease |
| III | <20% | <50% | Extensive FP or IP occlusions, alone or in combination with any disease in the other segment; popliteal CTO |
The GLASS system is primarily geared to correlate better with endovascular intervention outcomes and not surgical bypass outcomes. Factors that determine favorable anatomy for bypass surgery include adequate inflow and outflow and a suitable autologous venous conduit. Alternatively, the success of endovascular intervention mainly depends upon the complexity of atherosclerosis affecting the defined TAP. In addition, the GLASS system does not subsume factors like venous conduit quality or distal runoff that are integral for bypass grafting. Therefore, the preferred TAP for endovascular intervention and the preferred target artery for open bypass surgery may not always be the same.
Surgical Revascularization Procedures
Bypass surgery has been the historical treatment modality in most patients in need of lower extremity revascularization, but the less invasive nature of endovascular approaches and better outcomes data are shifting this paradigm. The change was reflected in the 2007 Trans-Atlantic Inter-Society Consensus (TASC II) document that expanded recommendations for endovascular therapies. TASC II classification of diseases of the FPS is shown in Table 14.4 . Although TASC class A and B lesions in symptomatic patients are best treated endovascularly, the recommended treatment for class D patients is surgical bypass in candidates suitable per these guidelines. Class C patients represent the “gray area” where endovascular techniques are applicable in selected patients not suitable for or agreeable to surgical bypass. These recommendations were based on the consensus of a panel of experts after reviewing the literature. For comparison purposes, the surgical gold standard for treating the diseased FPS is femoral above-knee popliteal venous bypass with 6-month, 1-, 2-, 3-, and 4-year primary patency rates of 87%, 81%, 77%, 71%, and 70%, respectively, with these numbers slightly decreasing when prosthetic material is used as the bypass conduit (85%, 77%, 66%, 59%, and 51% at the same time intervals).
| TASC II Group | Stenosis | Occlusion | Involvement of Infrageniculate Popliteal Artery | Heavily Calcified | Continuous Tibial Artery Runoff |
|---|---|---|---|---|---|
| A | Single <10 cm | Single <5 cm | OK | No | Yes |
| B | Multiple <5 cm | Multiple <5 cm | OK | No | Yes |
| Single <15 cm | No | No | Yes | ||
| Single popliteal stenosis | OK | No | Yes | ||
| Single or multiple lesions | OK | No | No | ||
| Single lesion <5 cm | OK | Yes | Yes | ||
| C | Multiple stenoses or occlusions >15 cm | OK | Yes or No | Yes | |
| Recurrent stenoses or occlusions requiring more than two endovascular treatments | OK | Yes or No | Yes or No | ||
| D | Chronic total occlusions of common and superficial femoral artery >20 cm | Yes | Yes or No | Yes or no | |
| Chronic total occlusion of popliteal artery and proximal trifurcation vessels | Yes | Yes or no | No | ||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree