Most of this chapter discusses management of occlusive disease of the subclavian and brachiocephalic arteries. Treatment of various conditions such as thoracic outlet syndrome and more distal lesions is also covered.
Subclavian and Brachiocephalic Disease
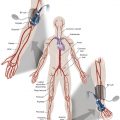
Symptomatic chronic ischemia of the upper extremity is commonly encountered in clinical vascular practice, comprising about 17% of symptomatic extracranial cerebrovascular disease ; 80% occurs in males. In contradistinction to chronic lower extremity ischemia, presenting symptoms in the upper extremity are often due to remote ischemia in the bed supplying collateral flow—namely, vertebral-subclavian and coronary-subclavian steal. However, ischemic symptoms of the upper extremity may occur with overutilization or hyperactivity of that extremity. This intimate association between the vascular territories of the arm, hindbrain, and heart (in post left internal mammary artery [LIMA] coronary artery bypass graft patients) is unique and may present technical challenges and potential complications not typically encountered in other vascular beds. With modern techniques and equipment, however, operators treating underlying stenotic or occlusive lesions of the proximal upper extremity arteries by interventional techniques can expect high degrees of success with low complication rates. , , ,
Indications
- •
Symptomatic chronic upper extremity ischemia
- •
Symptomatic vertebral-subclavian steal syndrome
- •
Symptomatic coronary-subclavian steal syndrome
- •
Asymptomatic subclavian stenosis or occlusion in a patient about to undergo coronary artery bypass graft with LIMA engraftment
- •
Preservation of dialysis or other vascular access
Contraindications
- •
Asymptomatic disease (in general, except as noted earlier)
- •
Renal insufficiency, severe aortic arch atherosclerosis (relative)
Equipment
- •
6F to 9F sheaths, 45 and 90 cm, long enough to extend through the area of intervention from the access site; 4F to 5F sheaths may be employed when using retrograde radial access or brachial access.
- •
Straight and angled hydrophilic wires
- •
Diagnostic catheters of various shapes, such as Judkins right (JR)4 (Cordis Corp., Miami Lakes, FL), multipurpose, headhunter, LIMA, VTK (Cook Medical, Bloomington, IN), Amplatz (Boston Scientific, Natick, MA), Kumpe (Cook Medical), and Simmons (Merit Medical, Salt Lake City, UT)
- •
Straight or angled-tip glide catheter
- •
0.035-inch exchange wire, atraumatic, straight, or J tipped; 0.014-to 0.018-inch guidewires for low-profile systems
- •
Balloons (including drug-eluting balloons) 4- to 10-mm diameter, 2 to 4 cm in length, with a long shaft length (110 cm long), if the site of access is from the femoral artery
- •
Balloon-expandable stents (including drug-eluting stents) capable of achieving these diameters, and lengths from 20 to 40 mm
- •
Covered stents in similar sizes for treatment of restenosis or for emergency use in vessel perforations
- •
Coronary stents in 3.0, 3.5, and 4.0 mm for vertebral or LIMA salvage (with compatible 0.014-inch wire)
Technique
Anatomy and Approaches
Symptomatic disease of the left subclavian artery is roughly eight to ten times more frequent than in the brachiocephalic trunk or right subclavian artery. , This can be considered fortunate because intervention in the brachiocephalic or right subclavian artery naturally involves working close to the right common carotid artery, potentially subjecting it to embolization, dissection, ostial compression, or stent coverage.
Proper technique, as always, begins with case planning and selection of vascular access. The strength of the indications must be weighed against the relative contraindications (severe generalized arch disease, renal insufficiency, long occlusions) and operator experience for good case selection. Most complications will happen early in an operator’s experience in more difficult cases.
Aortoostial flush occlusions, long occlusions, and lesions close to the vertebral, right common carotid, or internal mammary artery (IMA) are all “yellow flags,” to be given thoughtful consideration.
The operator should select the vascular access point that yields the highest chance of success with the lowest risk of complication at both the access and intervention site. In general, this is femoral access. Brachial access should be considered first in cases with heavily diseased or tortuous iliofemoral vessels and in severely tortuous aortic arches, particularly those with flush occlusions. Brachial access is also often used when guidewire access cannot be gained in antegrade fashion from a first femoral approach. In such cases, retrograde passage of the guidewire is often surprisingly easy. More recently, retrograde radial access has been commonly employed.
Technical Aspects
After vascular access has been selected and gained, a diagnostic catheter is selected based on the angulation involved and the need for coaxial support.
From a femoral or brachial access, a simple Kumpe, JR4, or IMA catheter may suffice for guiding a wire through a stenotic lesion; an occlusion, especially approached from the arch, may need the additional backup support offered by a VTK, Amplatz, or Simmons-shaped catheter. Great care must be used with such aggressive catheter shapes to avoid disrupting aortic plaque. When tempted to use them, brachial access should at least be considered as an alternative approach. Flow in the left vertebral artery is often retrograde, which adds a level of safety when dealing with potential embolization into the vertebral vascular systems. This should always be confirmed before intervention. Appropriate wire selection may include an 0.014- to 0.035-inch transition wire, a Wholey wire, or other similar metallic wire for stenosis, but a hydrophilic straight or angled wire will be necessary to cross most occlusions. Careful fluoroscopic guidance with road map support (if available) is necessary to ensure wire passage safely into the lumen beyond the occlusion. Care must be taken, especially with hydrophilic wires, to avoid propagating a dissection plane, either toward the vertebral artery or into the aorta retrogradely. Although helpful in infrainguinal revascularization, a subintimal approach in brachiocephalic disease is best avoided.
In occlusive disease, once the hydrophilic wire is free in the distal lumen, it is advisable to pass a diagnostic catheter (hydrophilic if necessary) through the occlusion, then remove the wire and confirm luminal placement by blood aspiration and gentle injection of contrast medium. A stiff exchange-length guidewire is then advanced through the catheter to complete the procedure.
Although there is no conclusive evidence in favor of primary stenting for subclavian and brachiocephalic artery lesions, most operators treat these lesions by stenting in preference to angioplasty alone.
Once guidewire access across the lesion has been achieved, one has the choice of whether to predilate before stent placement. Predilation has several advantages, including better visualization of the lesion length, especially of the distal stent landing point, proof of distensibility (especially in calcific lesions), and the ability to visually confirm correct balloon size. The main disadvantages are the possibility of propagating a dissection and, in theory, relieving the subclavian gradient enough to restore antegrade vertebral flow and lose the inherent protection against vertebral embolization retrograde flow provides. There is controversial evidence from a small study published in 1984 that restoration of antegrade flow may take several minutes, perhaps owing to chronic ischemic vasodilation in the arm. In our experience, however, restoration of antegrade flow in the vertebral artery occurs immediately on relief of the pressure gradient across the lesion, with either aggressive predilation or stenting. There is some evidence that direct stenting (i.e., without predilation) may be associated with a lower restenosis rate as well. In general, we gently predilate occlusions with undersized balloons to allow some antegrade flow for better visualization, and primary stent most stenoses that are not heavily calcified.
As is the case with most aortic branch vessel disease, most subclavian disease is ostial or near ostial. This includes the right subclavian artery, where typically the lesion is at the brachiocephalic bifurcation. In general, these lesions have a high degree of resistance to dilation and subsequent recoil; accordingly, balloon-expandable stents are generally preferred to self-expanding stents or angioplasty alone. ,
When positioning the stent, it is important to fully cover the lesion, but every attempt should be made to leave the ostium of the common carotid, internal mammary, and/or vertebral artery uncovered. One should be mindful, especially if predilation was not performed, that the vertebral artery or IMA may not be visible owing to reversed blood flow. If any of these ostia become compromised, rescue angioplasty with or without small vessel or coronary-sized stent placement should be considered. In the case of the ostial right subclavian artery, consideration may be given to placing an additional guidewire in the proximal right common carotid artery in the event the ostium is severely compromised when the stent is deployed. This can be performed via a second access site or through a slightly upsized sheath.
Many cases of stent migration before deployment in the lesion have been reported. , This usually arises from either the stent edge catching in the lesion during attempted passage through the lesion, or the rear of the stent catching on the guiding catheter during attempted stent withdrawal if the stent cannot be passed across the lesion. In both instances, the stent may be loosened (“skimmed”) off the balloon and left free on the wire. Although there are several methods available to rescue this event, including safely deploying the stent in a more proximal vessel, this situation is best avoided. Stents that are factory-rather than hand-mounted may be more resistant to skimming, and the risk can be eliminated entirely by readvancing the dilator and sheath through the lesion before the stent is advanced. In this way, the stent can be positioned while it is still protected inside the sheath, which can subsequently be withdrawn, and the exposed stent deployed. Especially when using 6F guiding sheaths, the potential for distal embolization is minimized.
Finally, after stent deployment, repeat angiography to assess optimal sizing should be undertaken, and postdilation can be performed if necessary, aiming for no more than a 1:1 balloon size–to-vessel ratio.
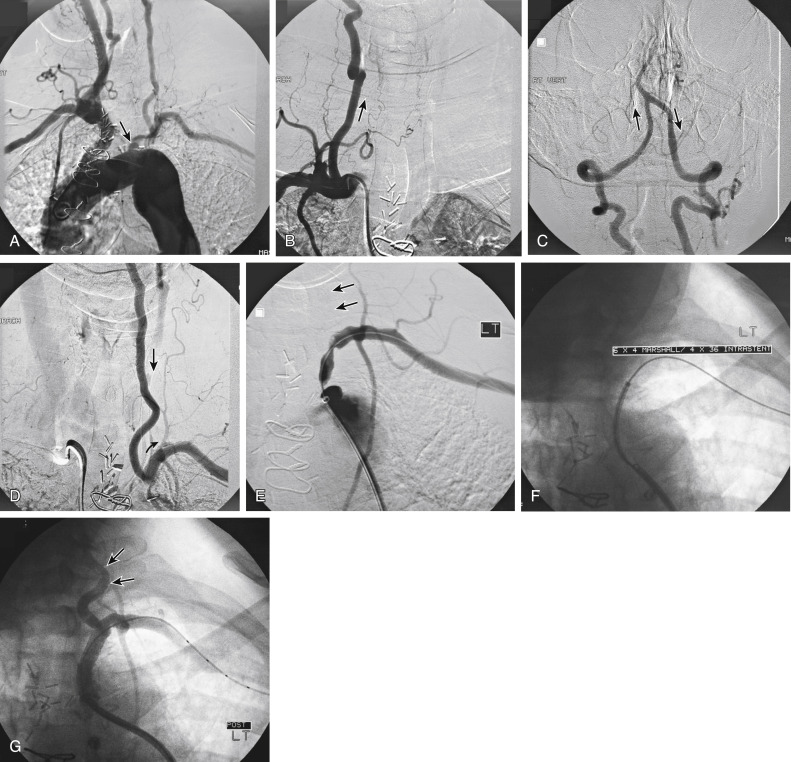
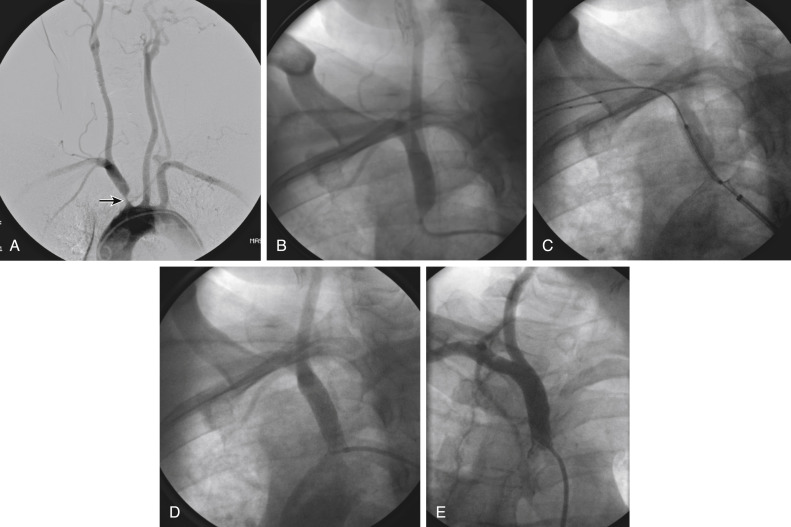
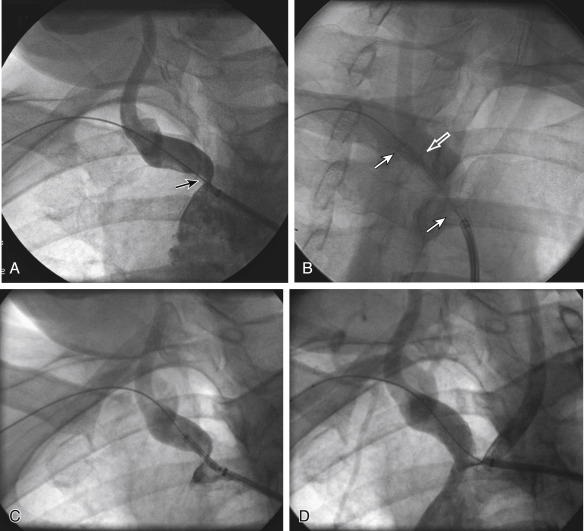
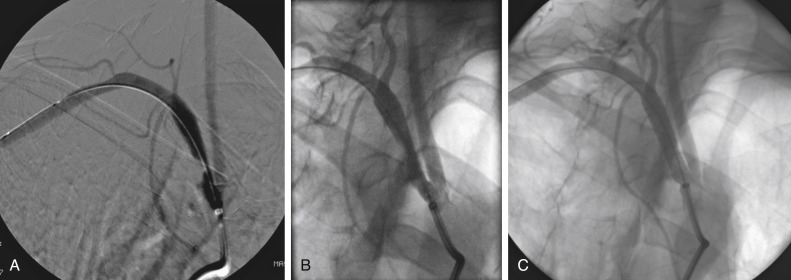
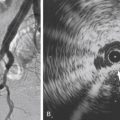
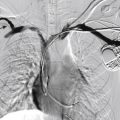
Figs. 12.1, 12.2, 12.3, 12.4, 12.5, and 12.6 illustrate various aspects of the interventions discussed.