Fig. 1
Bar diagram showing the approximate frequencies of different neuroendocrine tumors referred for 68Ga-SR PET/CT imaging at our center. The most common indication for referral has been gastroenteropancreatic tumors (GEP-NETs)
4 Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs)
Perhaps of all the NETs, tumors arising from the gastrointestinal tract are the most common. GEP-NETs have been traditionally classified into two main groups:
1.
Carcinoids
2.
Endocrine pancreatic tumors (EPTs).
The diverse and amorphous nature of GEP-NETs has led to a confused, overlapping, and changing terminology. In general, aggressiveness (malignancy), secretion (of hormones), and anaplasia (dissimilarity between tumor cells and normal cells) tend to go together, but there are many exceptions, which have contributed to the confusion in terminology. A proper understanding of the classification is thus important.
5 Classification
In 2000, the WHO introduced a classification system for NETs, which was updated in 2004 and most recently in 2010, differentiating between the terms NET and neuroendocrine carcinoma (NEC) (Kloppel et al. 2004). Important parameters in this classification include angioinvasion, proliferation index (Ki-67, MIB-1), and mitoses. Current classification of these tumors includes three categories and is presented in Table 1.
Table 1
WHO classification of neuroendocrine tumors. Also shown in the extreme right column is the grading system proposed by the European Neuroendocrine Tumor Society (ENETS)
Type | Size (cm) | Ki-67 index (%) | Tumor grade (proposed later by ENETS) |
|---|---|---|---|
Well-differentiated NETs | <2 | <2 | G1 |
Well-differentiated NECs | >2 | >2, angioinvasion | G2 |
Poorly differentiated NECs | >2 | >20 | G3 |
Although the WHO classification provides substantial information on tumor biology, complete prognostic assessment using these parameters is not enough. The most common NETs are the gastroenteropancreatic tumors (GEP-NETs), representing a heterogeneous group with different clinical behavior and prognosis. Hence, proper stratification is required to separate these tumors into different entities so that a group-selective treatment plan can be adopted. This prompted the European Neuroendocrine Tumor Society (ENETS) to issue guidelines on GEP-NETs (Rindi et al. 2006, 2007). A tumor–node–metastases (TNM) classification was later found to be clinically relevant by Fisher et al. (2008). In addition, a tumor grading system based on the mitotic count per square millimeter of tumor and the Ki-67 index has been proposed by ENETS (G1, G2, and G3) (Table 1).
6 Functional Imaging of GEP-NETs
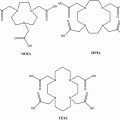
Primary neuroendocrine tumors (NETs) are often small and grow slowly. This often makes their identification and localization difficult. The introduction in 1994 of routine clinical use of single-photon emission computed tomography (SPECT) with metalloradiopharmaceutical 111In-diethylene-triamine-pentaacetic acid (DTPA)-octreotide (Octreoscan) spurred the development of somatostatin analogs labeled with therapeutic and PET radiometals. However, the acyclic bifunctional chelator (BFC) DTPA conjugated via one of the carboxyl group with the targeting vector molecule turned out to be unsuitable for use with radiometals other than In(III) because of its in vivo instability (Harrison et al. 1991). Consequently, a DOTA-substituted octreotide DOTA-d-Phe1-Tyr3-octreotide (DOTATOC) was synthesized (Heppeler et al. 1999). One of the most widely used macrocyclic frameworks to design BFCs is 1,4,7,10-tetraazacyclododecane (Mäcke and Good 2003). 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) is an octacoordinating ligand based on tetraazacyclododecane, in which each nitrogen atom bears an acetic substituent. Macrocyclic chelators provide exceptional inertness as well as high thermodynamic stability of the complexes. A disadvantage is the requirement for harsh labeling conditions (high temperature) to achieve high labeling yield because of the low kinetics of complex formation.
7 Studies with SRS (Somatostatin Receptor Scintigraphy)
111In-Pentetreotide (Octreoscan) has proven to be an important diagnostic tool for GEP-NETs (Lamberts et al. 1990, 1991; Krausz et al. 1998; Schillaci et al. 1997, 2000; Krenning et al. 1993). It has been reported to be more sensitive than conventional imaging modalities (CIM) by some authors (Krenning et al. 1993). Somatostatin receptor scintigraphy (SRS) with 111In-DTPA-octreotide however suffers from the drawbacks of the limited resolution of SPECT technology as well as unfavorable biodistribution in liver and spleen, obscuring small lesions.
8 Studies with 68Ga-Labeled Octreotide Analogs
Several different DOTA peptides (DOTATOC, DOTANOC, and DOTATATE) have been used in this clinical setting for either NET diagnosis or peptide-based radioreceptor therapy (PRRT). The utility of 68Ga-SR PET/CT in this regard ranges from diagnosis of disease extent and as a preliminary procedure to evaluate SSTR expression before the start of PRRT or cold somatostatin analog (SSA) treatment. Several studies in the past have established the efficacy of 68Ga PET/CT imaging in NETs with varying results (Srirajaskanthan et al. 2010; Ambrosini et al. 2010; Maecke et al. 2005; Gabriel et al. 2007).
At our center, the most important application of this radiotracer has undoubtedly been evaluation of patients with GEP-NETs. This is a well-established and regular procedure and penetrates into many aspects of GEP-NET management which include: (a) staging patients with already diagnosed NETs, (b) detection of sites of recurrence in patients with treated NETs (restaging), (c) diagnosis of patients suspected of having NET based on clinical features or biochemical evidence of hormone excess, (d) selection of potential candidates for cold SSA or PRRT, and (e) monitoring response to therapy in such patients.
9 68Ga-DOTATOC/NOC Synthesis
68Ga-DOTANOC synthesis was carried out by the Radiopharmacy Unit of the Nuclear Medicine Department, All India Institute of Medical Sciences (AIIMS), following procedures previously detailed by Zhernosekov et al. (2007) and briefly summarized here. A 30–50 mCi 68Ge/68Ga generator (Cyclotron Co. Ltd.; Obninsk, Russia) was eluted using 0.1 M HCl. The eluent was loaded on a miniaturized column of organic cation exchanger resin for preconcentration and prepurification (using 80% acetone/0.15 M HCl). The processed 68Ga (half-life, 68.3 min; β+ 88%; E β+ maximum, 1.9 MeV) was directly eluted with 97.7% acetone/0.05 M HCl into the reaction vial containing 30–50 μg DOTANOC/TOC. Synthesis was carried out at approximately 126°C for 10–15 min. This was followed by removal of labeled peptide from unlabeled peptide using reversed-phase C-18 column, using 400 μL ethanol. This was further diluted with normal saline and passed through 0.22-μm filter to obtain sterile preparation for injection. Labeling yield >95% was achieved within 10–15 min.
10 68Ga-DOTA-Peptide Imaging Protocol
68Ga-DOTANOC PET/CT was performed on a dedicated PET/CT unit (Biograph 2, Siemens Healthcare). Somatostatin analog therapy was stopped prior to PET/CT: short-acting analogs for 3 days before PET/CT and long-acting analogs for 4–6 weeks. Fasting was not mandatory. A dose of 132–222 MBq (4–6 mCi) of 68Ga-DOTANOC was injected intravenously (IV). After a 45- to 60-min uptake period, patients underwent PET/CT. No IV contrast agent was used. In the PET/CT system, the CT acquisition was performed with slice thickness of 4 mm and pitch of 1 on a helical dual-slice CT unit. Images were acquired using a matrix of 512 × 512 pixels and pixel size of 1 mm. After the CT acquisition, the table was moved toward the field of view (FOV) of PET, and PET acquisition of the same axial range was started with the patient in the same position. The PET components of the PET/CT system are based on a full-ring lutetium oxyorthosilicate PET system. Three-dimensional PET acquisition was performed from the base of the skull, including the pituitary fossa, to the mid thighs. Additional spot views were obtained when necessary. PET data were acquired using a matrix of 128 × 128 pixels and slice thickness of 1.5 mm. CT-based attenuation correction of the emission images was used. PET images were reconstructed by iterative method using ordered subset expectation maximization (two iterations and eight subsets). After completion of PET acquisition, the reconstructed attenuation-corrected PET images, CT images, and fused images of matching pairs of PET and CT images were available for review in the axial, coronal, and sagittal planes, as well as in maximum-intensity projections and in 3D cine mode.
11 Normal Biodistribution of 68Ga-Labeled Somatostatin Peptides
68Ga-DOTANOC is a tracer for somatostatin receptor-based PET imaging. It has predominant affinity for somatostatin receptors 2, 3, and 5 (SSTR2, 3, 5) (Kaemmerer et al. 2011). Hence it is physiologically distributed in organs which normally express high levels of SSTRs. It is important to be aware of the physiologic distribution of this tracer before attempting to interpret the pathologic sites of uptake. The highest uptake of 68Ga-DOTANOC is seen in the spleen, which is often considered the reference organ on 68Ga-DOTANOC PET/CT imaging (Fig. 2). Uptake in the pituitary and adrenal glands is also physiologic and constant. Mild uptake is seen in the liver and the thyroid gland, although uptake in the thyroid gland is quite variable. Mild, variable uptake has also been reported in the pancreas, especially the uncinate process (Castellucci et al. 2011). Physiologic excretion through the kidneys and the urinary bladder makes these organs evident on the PET/CT scan.
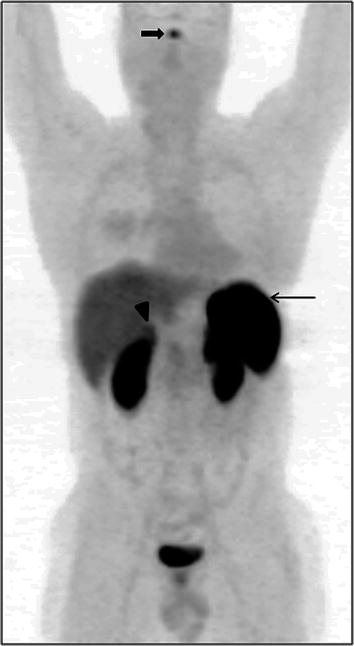

Fig. 2
68Ga-DOTANOC whole-body maximum-intensity projection (MIP) image showing the biodistribution of the radiotracer. Spleen shows the highest uptake on PET imaging and can be considered as the reference organ (arrow). Physiological uptake is seen in the pituitary (bold arrow) and adrenal glands (arrowhead). Mild uptake is seen in the liver, while excretion of the tracer through the urinary tract renders visualization of kidneys and urinary bladder evident
12 Sporadic GEP-NETs
The average sensitivity and specificity of 68Ga-DOTANOC PET/CT imaging for GEP-NET evaluation have been 78.2 and 92.5%, respectively, for detection of primary tumor (Fig. 3). These values are rather similar to those previously described in previous studies with this agent (Gabriel et al. 2007). Most disappointing has been however detection of biochemically confirmed diagnosis of insulinoma, with a detection rate of only 18%. This limitation of SRS-based imaging has been previously acknowledged (Zimmer et al. 1996). Uptake analysis has shown that pancreatic tumors have higher uptake than other primary sites. This might be because of the fact that pancreatic NETs are usually well differentiated with higher expression of SSTR2A receptor (Prasad et al. 2010; Campana et al. 2010) (Fig. 4). One of the important concerns in this aspect has been the occasional high degree of uptake in the pancreatic head, which can give rise to false positives leading to unnecessary surgical procedures. Recent reviews routinely mention this finding of increased uptake in the head of pancreas as physiological (Ruf et al. 2011). However, this problem has not been a concern of late with increased experience in reporting such studies and was therefore a part of the preliminary hardships. The average sensitivity and specificity of 68Ga-DOTANOC PET/CT for detecting metastatic disease are 97.4% and 100%, respectively. The high rate of positivity obtained with in vivo 68Ga-DOTANOC PET/CT is not surprising since high-affinity somatostatin binding sites have been found in vitro on most GEP endocrine tumors (Ruf et al. 2011; Reubi et al. 1991). The majority of these tumors contain high numbers of SSTRs, homogeneously distributed throughout the tumor and expressed at both primary and metastatic sites (Reubi et al. 1991) (Fig. 5).
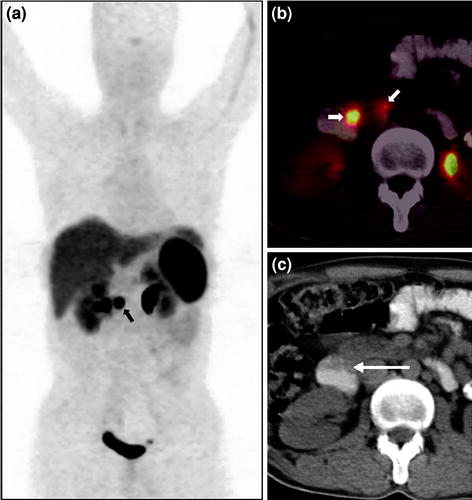
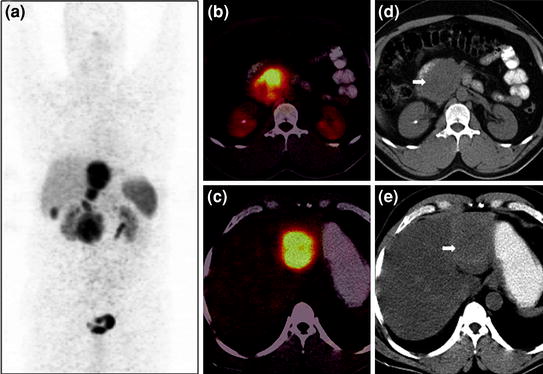
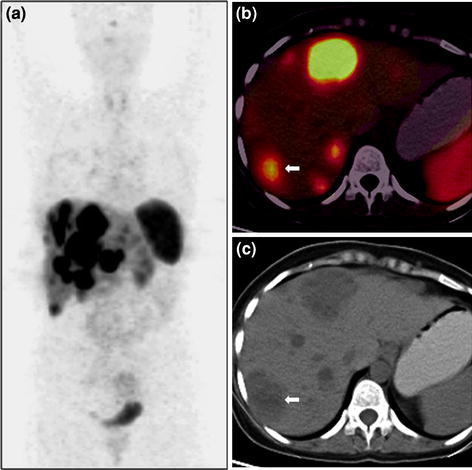

Fig. 3
A 38-year-old male with clinical features suggestive of gastrinoma and elevated serum gastrin level (1,400 pg/ml) who presented with no apparent abnormality on conventional contrast-enhanced CT (CECT) and a suspicious lesion in the duodenum on upper-GI endoscopy underwent 68Ga-DOTANOC PET imaging for confirmation of diagnosis and localization of a possible source. Whole-body PET MIP image shows a focal area of increased tracer uptake in the abdomen (a, bold arrow). Axial PET/CT image (b) shows two focal areas of uptake in the region of duodenum (bold arrows), later found to be the primary tumor in second part of duodenum, and a small regional lymph node confirmed to be well-differentiated NET on histopathological analysis. 68Ga-DOTANOC PET/CT not only helped in confirming the diagnosis but also revealed the involved regional node, removal of which reduces the chances of recurrence in this case. Axial unenhanced CT shows no apparent abnormality in the duodenum (c, arrow)

Fig. 4
A 50-year-old male with well-differentiated neuroendocrine carcinoma of pancreas with liver metastases underwent staging 68Ga-DOTANOC PET/CT imaging. Whole-body PET MIP image shows intense tracer uptake in the abdomen at multiple locations (a). Axial PET/CT images show increase tracer uptake in the pancreatic head (b) and liver (c). Corresponding unenhanced CT images show a homogeneous soft tissue mass involving the head of pancreas (d, bold arrow) and liver lesion on a fatty liver background (e, bold arrow). The patient is under treatment with 177Lu-DOTATATE PRRT therapy and shows stable disease on follow-up imaging

Fig. 5
A 35-year-old female with carcinoid tumor of colon (post surgery) and multiple liver metastases underwent restaging 68Ga-DOTANOC PET/CT imaging to explore the possibility of octreotide therapy. 68Ga-DOTANOC whole-body maximum-intensity projection (MIP) image revealed intense tracer avidity in the liver lesions (a) shown on PET/CT (b) and unenhanced CT images (c). Patient underwent therapy with long-acting octreotide analog (Sandostatin) and is stable 6 months post therapy on follow-up PET imaging
This has been the best modality to detect metastases in lymph nodes and bones (Putzer et al. 2009), including at our center, leading to comprehensive whole-body assessment and subsequent therapy planning. Liver is the most common site of metastases of GEP-NETs. Although no significant difference is seen in the evaluation of liver lesions when compared with CT or MRI, it is important from a prognostic point of view. Patients with documented liver metastases routinely undergo 68Ga-DOTANOC and 18F-FDG (fluorodeoxyglucose) PET/CT at our center, if possible. This often helps in selecting the best possible treatment for such patients, as a combination of these two imaging modalities is known to help classify these tumors into appropriate differentiation categories with an acceptable degree of accuracy. Well-differentiated tumors are mostly treated with octreotide-based regimes, while less-differentiated neoplasms are known to respond to combined chemotherapy using etoposide and cisplatin (Reubi et al. 1991; Moertel et al. 1991; Hainsworth et al. 2006).
13 Metastatic GEP-NETs with Occult/Uncertain Primary Tumor
Neuroendocrine tumors account for about 2–4% of carcinoma of unknown primary site (CUP) and are often mentioned separately because this entity belongs to a treatable subset (Hainsworth et al. 2006). Identification of the primary site is of prime importance as many aspects of tumor management are dependent on it, ranging from disease prognosis, treatment outcomes, to survival rates. Although detection of the primary tumor in patients with metastatic NET may not change the disease stage, its detection and removal can prevent the patient from having significant local symptoms; e.g., small bowel NETs are notorious for causing desmoplastic reaction and adjacent fibrosis which can cause obstructive features. SRS using 111In-DTPA-octreotide has also been explored to detect occult primary sites in patients with metastatic GEP-NETs with a detection rate of 39% (Savelli et al. 2004). The role of PET/CT using 68Ga-labeled somatostatin analogs to localize the primary tumor in patients with CUP-NET was first assessed by Prasad et al. (2010). They demonstrated that 68Ga-DOTANOC PET/CT was able to localize the primary tumor in 59% of the patients enrolled, with an overall change in management in 10% of cases. A study from our center has found similar results, although the number of patients was less compared with their study (Naswa et al. 2012). A detection rate of 60% has been observed in our series, with midgut being the most common site of primary tumor localization (Fig. 6). In patients where no primary could be localized, several hypotheses can be put forward as explanations. First, there may be receptor downregulation which hinders tracer binding and detection. The size of the tumor might be too small to be detected by the resolution of the imaging system. Finally, dedifferentiation of tumor cells is another possibility, causing failure to detect on 68Ga-DOTANOC PET/CT, giving way to 18F-FDG-PET/CT imaging in this regard. However, the role of 18F-FDG-PET/CT still remains to be explored in this subset of patients, as the low metabolic rate and indolent nature of NETs might cause too many false-negative results (Adams et al. 1998). Even in patients in whom no primary tumor is localized, additional sites of metastatic disease are observed when compared with conventional imaging, the most common being lymph nodes and bone. Although detection of such additional metastatic lesions do change management in these patients, they are certainly important from a prognostic point of view.
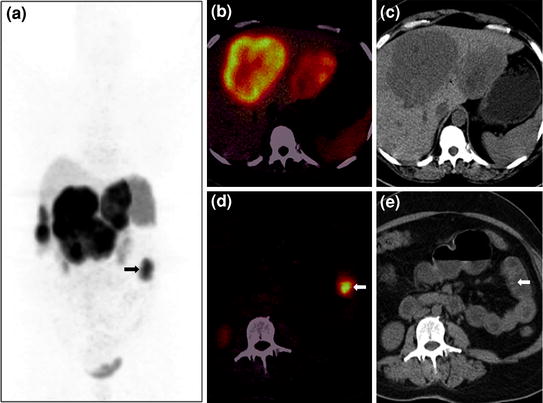

Fig. 6
A 46-year-old female with NET metastases to liver and a suspected primary tumor in the small bowel underwent 68Ga-DOTANOC PET/CT imaging. Whole-body PET MIP image shows multiple areas of increased tracer uptake in the liver suggestive of liver secondaries and an area of abnormal uptake in the left side of abdomen (a, bold arrow). Axial PET/CT image (b) reveals increased tracer uptake in the liver, while corresponding CT image (c) shows the large hypodense lesions with a necrotic center. A focal area of uptake is seen in a jejunal loop on PET/CT (d), while no definite abnormality is seen on the CT section (e, bold arrow)
14 Gastroenteropancreatic Neuroendocrine Tumors in Patients with Hereditary Syndromes
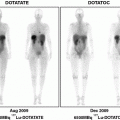
The most common hereditary cancer syndrome associated with GEP-NETs is the multiple endocrine neoplasia (MEN1) syndrome. GEP tumors associated with MEN1 syndrome are usually functional and commonly include gastrinomas (60%) and insulinomas (10%), although carcinoid tumors are also known to occur (Thakker 2000). Most of these tumors belong to the category of well-differentiated neuroendocrine tumors and are known to express SSTR on tumor cell surface (Kaltsas et al. 2004). Functional imaging with 111In-DTPA-octreotide is a proven method to image neuroendocrine gastroenteropancreatic tumors, but currently lacks full evaluation in MEN1 syndrome, including its usefulness for early diagnosis of tumors (Shi et al. 1998). However, it is considered to be the procedure of choice for screening endocrine tumor metastases in MEN1 patients (Langer et al. 2004). High degree of uptake on SRS imaging (both SPECT and PET) has been previously mentioned by Ezziddin et al. (2006) and Campana et al. (2010) in their studies. However, they could not find any significant difference in tracer uptake of functioning and nonfunctioning NETs. A higher degree of 68Ga-DOTANOC uptake, however, has been shown to have a better long-term outcome than tumors with low maximum standardized uptake value (SUVmax) values even in patients with advanced stage of the disease (Campana et al. 2010). Indeed in our series of seven such patients, the median SUVmax of the detected lesions was 36.1, with all of them showing stable disease after median follow-up of 2 years, clinically and radiologically (Fig. 7).
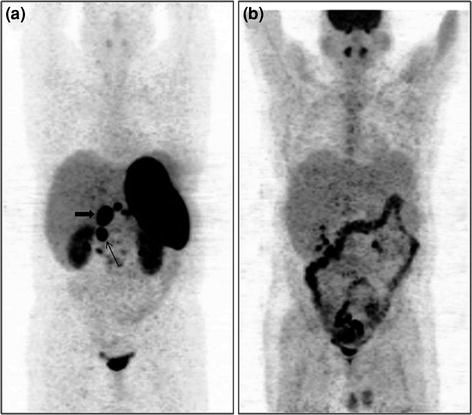

Fig. 7
A 32-year-old male with MEN1 syndrome had presence of a pyloric tumor with regional lymph node involvement. 68Ga-DOTANOC whole-body MIP image shows abnormal areas of uptake in the abdomen (a, bold arrow-primary tumor, arrow-regional lymph node). 18F-FDG PET MIP image (b) shows normal distribution of tracer with no abnormal uptake within the abdomen. Histopathology (HP) analysis of the primary tumor revealed features of a well-differentiated NET with Ki-67 index <1%
SUVmax is a semiquantitative parameter of tumor uptake and has now been proven to be an important and independent prognostic factor in the natural history of patients with neuroendocrine tumors. Campana et al. (2010) prospectively investigated the prognostic value of 68Ga-DOTANOC SUVmax in 47 NET patients and found that SUVmax was significantly higher in patients with well-differentiated NETs. Among primary tumor sites, pancreas had higher uptake than other tumor sites. Patients with higher tumor SUVmax values showed a stable pattern of disease or partial remission on follow-up, with a cutoff value of 17.9–19.3 being the best prognostic indicator of favorable prognosis. Tumors with lower SUVmax progressed more often. Results from our center are in agreement with this observation. The average SUVmax values of different tumors and sites are shown in Table 2. However, among the primary tumors, histological subtypes do not influence the uptake of 68Ga-DOTANOC. No significant difference is observed in the SUVmax of carcinoid with gastrinoma and NET not otherwise specified (NOS) (Naswa et al. 2011).
Table 2
Results of semiquantitative analysis on 68Ga DOTANOC PET/CT
Site | SUVmax, median (range) |
|---|---|
Primary (all) | 13 (1–125) |
Carcinoid | 13 (3.3–125) |
Gastrinoma | 11 (4.5–58.2) |
Insulinoma | 29.3 (23.5–48.3) |
NET (NOS) | 11.3 (1–69) |
Metastasis (all) | 14.5 (1.3–145) |
Liver | 16.8 (4.3–145) |
Lymph node | 9.6 (2.3–50) |
Bone | 5.5 (1.3–53) |
15 Correlation of Tumor Uptake with Cellular Differentiation
Uptake of somatostatin analogs has been shown to be dependent on a number of variables (Ezziddin et al. 2006). Perhaps the most important amongst these is the degree of cellular differentiation. The system recently proposed for gastroenteropancreatic NETs by the ENETS and also now recommended by the WHO uses either mitotic rate or Ki-67 labeling index (Rindi et al. 2007) (Table 1). Ki-67 is a nuclear antigen expressed in proliferating cells (G1, S, G2, and M phases) but not in resting cells (G0 phase). Cell proliferative activity is an important indicator of the growth and behavior of various human malignant tumors, because tumors with higher Ki-67 expression are associated with poorer prognosis (Lee 1996). Kimura et al. have shown that Ki-67 is an excellent indicator to assess progression of neuroendocrine tumors. Tumors with high Ki-67 values (>50 labeled nuclei/field) progress rapidly with the worst outcome; tumors with moderately high Ki-67 values (20–50 labeled nuclei) progress slowly but with poor outcome; and tumors with very low Ki-67 values (<10 labeled nuclei) have no metastases and good prognosis (Kimura et al. 1994). Belhocine et al. in their study on 17 patients with carcinoid tumors, correlated findings on somatostatin receptor scintigraphy (SRS) with the histological characteristics of the lesion. They found low expression of Ki-67 (<10%) in all well-differentiated carcinoid tumors, although no clear relationship could be found between proliferative activity and SRS results (Belhocine et al. 2002). In another study, Adams et al. showed the linear relationship between higher proliferative rate and FDG uptake. They concluded that PET imaging of gastroenteropancreatic tumors reveals increased glucose metabolism only in less-differentiated GEP tumors with high proliferative activity and that additional 18F-FDG PET should be performed only if somatostatin receptor scintigraphy is negative (Adams et al. 1998). A comparison of 68Ga-DOTANOC and 18F-FDG studies done at our center in 26 patients has shown that well-differentiated GEP-NETs with low Ki-67 index have higher tumor uptake, while uptake on 18F-FDG PET is higher in poorly differentiated tumors. However, a level of statistical significance could not be reached in this comparison. This holds true for both primary and metastatic sites. Also tumors with higher SUVmax values tend to follow a stable pattern of disease over time, while tumors with lower uptake and low SUV values progress over time. Hence, tumor SUVmax values on PET/CT can be considered an in vivo proxy of the prognostic value which Ki-67 holds.
16 Correlation of Tumor Uptake with SSTR Subtypes
The effects of somatostatin and its analogs are mediated via specific cell membrane-bound high-affinity receptors located on target cells. Five subtypes of SSTRs, SSTRs 1–5, have been cloned, and they belong to a distinct group within the superfamily of G-protein-coupled receptors with seven transmembrane regions (Reisine et al. 1995). Of practical importance is the division of the five receptor subtypes into two groups, where SSTRs 2, 3, and 5 differ from SSTRs 1 and 4 regarding amino acid homology and pharmacological profile. Short somatostatin analogs bind to the first group of receptor subtypes. Octreotide has highest affinity to SSTR 2 and lower affinity to SSTRs 3 and 5. 68Ga-DOTANOC seems to be the most promising peptide for broad SSTR subtype affinity (binding to SSTR2, SSTR3, and SSTR5) (Wild et al. 2003). In a study by Miederer et al. in 18 patients, 68Ga-DOTATOC PET/CT scans were quantified by SUV calculations and correlated to a cell membrane-based SSTR2-immunohistochemistry (IHC) score (0–3). They found that negative IHC scores were consistent with SUV values below 10, and all scores of 2 and 3 specimens corresponded with high SUV values (above 15). This validates the use of SSTR2 IHC in patients missing a preoperative PET scan to indicate 68Ga-DOTA PET/CT as a method for restaging and follow-up in individual patients (Miederer et al. 2009). IHC correlation of SSTR subtypes with tumor uptake is however not available at our center.
17 Impact on Management
It is evident from the discussion above that 68Ga-DOTANOC is clearly superior to any other currently available imaging modality in detection of gastroenteropancreatic neuroendocrine tumors. The main question in this regard, however, is whether detection of a greater number of lesions transforms into an overall survival advantage and creates a change in the management proposed. Ambrosini et al. were first to demonstrate that 68Ga-DOTANOC PET/CT either affected stage or caused a therapy modification in more than half the patient population studied (n = 90; 55.5%), confirming the clinical role of 68Ga-DOTANOC PET in management of NETs (Ambrosini et al. 2010). A study of 109 gastroenteropancreatic tumors at our center showed an overall influence in almost half of the patients (48%) regarding management decisions (change in 19%, support in 29%) (Naswa et al. 2011). The main advantage of PET in this regard is its impact on management decisions, rather than a change in stage. Important in this regard is either the inclusion or exclusion of surgical procedures. Also, in vivo demonstration of SSTR expression offers the physician a chance to treat these tumors with octreotide analogs, both cold SSA analogs and peptide-based radionuclide therapy (PRRT). Treatment with both these agents has shown to reduce signs and symptoms of hormone hypersecretion, to produce an overall improvement in quality of life, and to slow tumor growth, thus conferring a survival advantage to the patient (Bodei et al. 2009). It is also important in this regard to understand that a negative finding (no tracer uptake) on 68Ga-DOTANOC PET in a demonstrated morphologic abnormality is as important as its ability to detect true-positive lesions. Targeted therapy would not be an effective therapy for such patients but rather than a switch to chemotherapy will provide a better option; hence, 68Ga-DOTANOC PET becomes a mandatory procedure in patients with GEP-NETs to select the appropriate treatment strategy (Moertel et al. 1991).
18 Role of FDG-PET/CT
The workhorse of PET imaging, 18F-FDG, exploits the increased glycolytic activity of tumor cells, which appears lacking in most NETs (Hawkins 1995; Kayani et al. 2008). This appears to be the predominant reason for the limited sensitivity of 18F-FDG in this category of tumor and has been repeatedly mentioned in the literature (Adams et al. 1998). The importance of 18F-FDG-PET imaging in GEP-NETs is, however, to target a different issue: detection of tumor cells with increased metabolic activity, which has an impact on prognosis and management (Strauss and Ponti 1991). A total tumor population characterization using a combination of 18F-FDG and 68Ga-labeled octreotide analog therefore seems a plausible approach (Fig. 8). This can map the entire extent of tumor cell differentiation in the same patient (Kayani et al. 2008), detect occult primary tumors as well as metastatic sites (which can change management), select the appropriate mode of treatment based on predominant cellular differentiation (somatostatin therapy for well-differentiated and conventional chemotherapy for less well-differentiated tumors), and select potential candidates for undergoing peptide-based radionuclide therapy in GEP-NETs when they are surgically unresectable. Hence, 18F-FDG PET/CT must be considered complementary to 68Ga-DOTANOC PET imaging, rather than contrasting (Binderup et al. 2010).
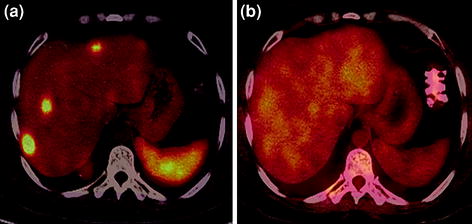

Fig. 8
A 35-year-old male with well-differentiated NET (gastrinoma) underwent restaging PET/CT. Increased tracer uptake is seen in the multiple liver metastases on 68Ga-DOTANOC PET/CT axial image (a) with no avidity to FDG on 18F-FDG PET/CT imaging (b)
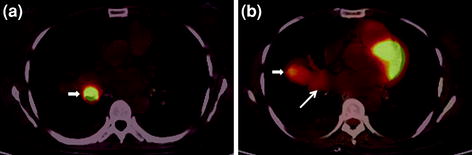
19 Pulmonary Neuroendocrine Tumors
The recent WHO classification of pulmonary neuroendocrine tumors classifies these neoplasms in order of increasing malignant potential into typical and atypical carcinoids, and large cell and small cell neuroendocrine tumors (Brambilla et al. 2001). Patients with pulmonary neuroendocrine tumor often present with recurrent pneumonia, cough, hemoptysis, or chest pain. Most pulmonary carcinoids are typical carcinoids with metastases in only 15% and a high 5-year survival rate of over 90% (Morandi et al. 2006). They are 3–4 times more common than atypical carcinoids, which tend to occur in older patients, are usually larger, and more often arise peripherally. They metastasize in about 30–50% of cases with a 5-year survival rate of 40–60% (Srirajaskanthan et al. 2008). Pulmonary carcinoids, especially the typical variety, are considered indolent with a very low metabolic rate and express a very high density of somatostatin receptors at the cell surface. Various studies in the past have explored somatostatin receptor-based PET/CT with 68Ga-labeled somatostatin analogs in pulmonary neuroendocrine tumors, often in conjunction with 18F-FDG PET, and most of them have reached a rather common opinion that typical bronchial carcinoids show a high degree of 68Ga-DOTA-octreotide uptake while high-grade atypical bronchial carcinoids tend to show less uptake (Figs. 9, 10). They are, however, intensely FDG avid (Kayani et al. 2009). A combination of the two functional imaging procedures can be used for presurgical planning in primary bronchial carcinoids and for restaging recurrent pulmonary neuroendocrine tumors. Similar results and experiences have been reported from our center in the past (Jindal et al. 2011).