SN
Receptor subtype
Native peptide
Origin
1
NMB-R or BB1
Neuromedin B
Mammalian
2
GRP-R or BB2
Gastrin-releasing peptide
Mammalian
3
BRS-3 or BB3
Bombesin
Mammalian
4
BRS-4 or BB4
Bombesin
Amphibian
The bombesin receptor family is receiving increased attention as a means of localizing tumors or other disease processes by receptor-mediated imaging (Okarvi 2008; Gonzalez et al. 2008; Tweedle 2009; Schroeder et al. 2010). Anastasi et al. (1971) initiated research into the 14-amino-acid peptide bombesin receptor system with the isolation of the liner amidated tetradecapeptide bombesin from the skin of the European frogs Bombina bombina, Bombina variegate, and Bombina orientalis (Erspamer et al. 1970; Erspamer 1988; La Bella et al. 2002; Jensen et al. 2008). Amphibian bombesin has various effects in mammals, and bombesin-like immunoreactivity has been noticed in mammalian brain, lung, and gastrointestinal (GI) tract including the pancreas (Ghatei et al. 1984). McDonald et al. isolated a 27-amino-acid peptide, using antisera developed against bombesin, from porcine stomach, homologous to the C-terminus of bombesin, which was named GRP after its first known activity of inducing gastrin secretion from G cells in the gastric antrum. GRP peptide shares the seven -COOH terminal amino acids with Bn (McDonald et al. 1979). The second mammalian bombesin-like decapeptide NMB was isolated by Minamino et al. (1983), from porcine spinal cord (McDonald et al. 1979). NMB is widely distributed in the brain and GI tract, and like GRP, shares six of the seven -COOH terminal amino acids with litorin (Minamino et al. 1983). A third bombesin-like peptide, neuromedin C (NMC or GRP-10), is identical to the carboxy-terminal subsequence (Jensen 1994; Ladenhein et al. 1994; Hajri et al. 1996; Kane et al. 1996; Kim et al. 1996; Koh et al. 1999) of GRP and has a potent contractile action on rat uterus in the characteristic manner of bombesin (Minamino et al. 1984).
Bombesin and GRP are known as brain–gut peptides and exert a variety of physiological actions in the nervous system and the gut. The normal physiological role of GRP in the human body is to stimulate secretions of gastrointestinal hormones and by the exocrine pancreas and to modulate gastrointestinal motility. Bombesin response appears to be central nervous system mediated, and its expression is not dependent on the vagus nerves or adrenal glands and does not rely on gastric secretion. The demonstrated central nervous system-mediated effects (Tachf et al. 1980) of bombesin include development of sustained hyperglycemia (Brown et al. 1979; Moore 1973) and hyperglucagonemia (Nemeroff et al. 1979), which is adrenal dependent through an increase in sympathetic outflux (Brown et al. 1979). It has been shown that hyperglycemia (Brown et al. 1979; Tachf et al. 1980), hyperglucagonemia (Lin and Warrick 1974; Christiansen et al. 1976), and increased sympathetic outflux (Osumi et al. 1977; Yamaguchi et al. 1977) singly decrease gastric acid secretion in experimental animals and in humans. Both peptides are good carriers for radiometals to target cancer, since their receptors are overexpressed in a number of cancer types. Since Bn is assumed to be more stable than GRP, developments have mainly been focused on Bn analogs. Cuttitta et al. (1985) demonstrated that Bn and GRP can stimulate small cell lung cancer growth (Reubi 2003), and the same were shown by the group of Rozengurt et al. (1990) to be mitogenic in Swiss 3T3 cells. They reported that Bn-like peptides could function as autocrine growth factors in small cell lung cancer (SCLC) (Cuttitta et al. 1985; Rozengurt 1990). It is known that SCLC cells produce BLPs and overexpress Bn/GRP (Cuttitta et al. 1985), forming an autocrine or paracrine loop to promote SCLC growth and invasion. A number of researchers using different methodologies to block either the BLPs or GRP-R have reported significant growth inhibition of SCLC in vitro as well as in vivo (Avis et al. 1991; Halmos and Schally 1997). GRP acts mainly in the central and enteric nervous systems, and it regulates several physiological processes including satiety, thermoregulation, and circadian rhythm, as well as release of other peptide hormones.
Bn and GRP share a seven-amino-acid sequence in their C-terminal regions that is essential for receptor binding and biological activity (Shipp et al. 1991; Cai et al. 1994; Faintuch et al. 2008). Since GRP mimics every effect of bombesin, GRP was initially considered to be mammalian bombesin, but GRP and bombesin are distinct peptides (Nagalla et al. 1992). Based on the fact that both GRP and bombesin are found in frog and on phylogenetic examination, it is suggested that the two peptides separated before the vertebrate radiation. The similar biological activity of the peptides is explained by the difference in only 1 of the 10 carboxy-terminal residues. The peptides were divided into three groups on the basis of the amino group: the bombesin-related peptides, the ranatesin peptide, and the phyllolitorin peptide (Erspamer et al. 1970; Erspamer 1988; Jensen et al. 2008) (Fig. 1).
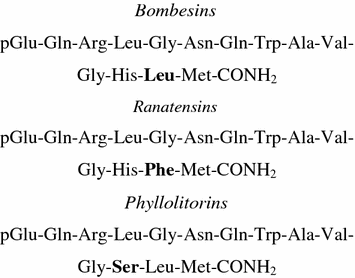
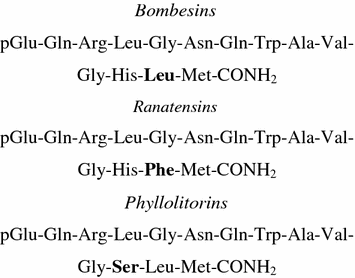
Fig. 1
Bombesin and related peptides
Ranatesin, an undecapeptide isolated from the skin of Rana pipiens, was also isolated from porcine spinal cord by using stimulation of rat uterine contractility as a bioassay by Minamino et al. (1983). The other analog is alytesin, isolated from Alytes obstetricians (Erspamer et al. 1970; Preston et al. 1996). All peptides are associated in sequence and share an amidated methionine at their carboxyl termini (C-termini), which characterizes the bombesin subfamilies and exhibits similar side-effects in pharmacological assays (Erspamer et al. 1970; Anastasi et al. 1971). The bombesins and ranatensins have leucine and phenylalanine as the penultimate residue, respectively, and the phyllolitorins have a serine as the third residue from the carboxyl terminus.
BLPs are neuropeptides found in both the central and peripheral nervous systems as well as in endocrine-like cells in several tissues including lung and thyroid (Lebacq-Verheyden et al. 1990; Sunday et al. 1988). They play important physiologic roles in regulating smooth muscle contraction, modulation of neuron firing rate (Lebacq-Verheyden et al. 1990 ; Spindel 1986), release of hormones in the gastrointestinal tract, and secretion of pancreatic enzymes, and serve as neurotransmitters in the central nervous systems (Bunnett 1994). Nerve fibers containing bombesin-like immunoreactivity are distributed in the intrinsic neurons of the stomach, pancreas, small intestine, and colon (Moran et al. 1988). BLPs have also been shown to stimulate proliferation of some GI tumors (Narayan et al. 1990), suggesting that these peptides may play a role in the development of cancers. However, it is not clear to what extent the growth-promoting effects of bombesin are due to a direct action on target cells or to the release of other growth factors (Upp et al. 1988; Liehr et al. 1992; Bunnett 1994). Bn/GRP-Rs are tied to G-protein through their intracellular domain and belong to the G-protein receptor (GPR-R/GPRR) superfamily. Bn/GRP-R multiple cellular signal transduction pathways are activated on binding, including protein kinase C (PKC), mitogen-activated protein kinase (MAPK), tyrosine phosphorylation of focal adhesion kinase (FAK), paxillin, p130cas, and calcium mobilization, resulting in cell proliferation and growth (Aprikian et al. 1997; Hellmich et al. 1999).
BLPs have also been shown to have mitogenic effects in a number of cancer cell lines (Bunnett 1994). Of these four receptors, three (BRS-3, NMB-R, and GRP-R) have been shown to be expressed to varying degrees in a variety of human tumors and human cancer cell lines (Reubi et al. 2002; Cornelio et al. 2007). For prostate and breast carcinomas, the GRP-R subtype has received the most attention from the research community due to the high expression density of the receptor relative to the NMB-R and BRS-3 subtypes (Gugger and Reubi 1999; Markwalder and Reubi 1999; Reubi et al. 2002). In normal human tissues, the pancreas has the highest degree of GPR-R gene expression, while the stomach, brain, and adrenal glands have notably lower levels (Jensen et al. 2008). The low expression of GPR-R in normal tissue relative to the increased expression in many cancers has validated GPR-R as a target for cancer detection and treatment. So far, a native peptide ligand showing high binding affinity to the BRS-3 subtype has not yet been identified. The Bn receptor family consists of three hepta-helical, guanine-nucleotide-binding regulatory protein (G-protein) coupled receptors, which are situated at the outer membrane of target cells and comprise three members in mammals: the 384-amino-acid GRP receptor (GRP-R) with high affinity for GRP, which has 55% amino acid identity to the 390-amino-acid neuromedin B receptor (NMBR) with high affinity for NMB, and the 399-amino-acid orphan BB3 receptor (BB3-R), bombesin receptor subtype 3 (BRS-3) (Okarvi 1999). The BRS-3 receptor is included in the mammalian Bn receptor family because it has 47–52% homology to GRPR and NMBR even though its natural ligand is still unknown (Bunnett 1994; Reubi 2003; Krenning et al. 2004). BRS-3 has a more limited distribution than GRPR and NMBR, but is found in both the central nervous system (CNS) and peripheral tissues, especially the GI tract (Okarvi 1999). The BRS-3 receptor has been replicate from human, mouse, and sheep, and tissue-specific expression has been reported (Whitley et al. 1999).
Bombesin initiates many of its biological effects by binding to and activating specific cell surface receptors which are coupled to phospholipase C signaling cascades via pertussis toxin-insensitive G-proteins (Fischer and Schonbrunn 1988; Rozengurt 1991; Bunnett 1994) and also activates a number of tyrosine kinase cascades (Okarvi 1999; Reubi 2003; Krenning et al. 2004). Many of the effects of bombesin are produced by the second messengers inositol 1,4,5-trisphosphate and diacylglycerol (Swope and Schonbrunn 1988; Rozengurt 1991; Bunnett 1994) leading to intracellular calcium mobilization (Kroog et al. 1995; Ryan et al. 1998). Amphibian bombesin exhibits high affinity for all the receptor subtypes (Erspamer 1988; Kroog et al. 1995). More specifically, GRP, which is an agonist, is endocytosed following receptor binding. The receptors are either recycled to the cell surface or degraded, while the ligand remains in the perinuclear space.
3 Targeted Approach to Receptor-Mediated Imaging
The identification of various Bn receptors and their receptor-mediated imaging have prompted the development of a wide variety of GPR-R targeted diagnostic imaging, chemotherapy, and radiotherapy agents. Radiolabeled GRP remains in the target tissue for longer periods of time, compared with radiolabeled antagonists, resulting in higher accumulation of radioactivity in GRP-R-positive tissues (Van de Wiele et al. 2001). Upon binding of the agonist bombesin analog to the GPR-R, the peptide and the attached chemotherapy agent and/or radionuclide are internalized and thereby retained in the cell. The bifunctional chelate design has primarily been used in the development of GPR-R-targeted Bn analogs for nuclear diagnostic imaging and radiotherapy. This approach has four distinctive components: a targeting vector, a radiometal, a chelation system, and a linking group (Fig. 2).
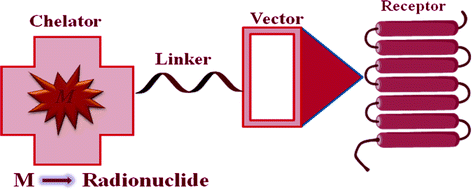

Fig. 2
Schematic representation of receptor-mediated approach
3.1 Why Bombesin Receptor-Mediated Imaging?
3.1.1 A Targeting Vector
The choice of targeting vectors for bombesin analogs has varied from the use of the entire 14-amino-acid bombesin peptide to truncated portions of the peptide, with the latter being most prevalent in the literature. In any event, the targeting vector must employ the Bn (8–14)NH2 or a GPR-R-targeted derivative of this amino acid sequence in order to achieve uptake in GPR-R-expressing tissues. Novel pharmacological insights regarding bombesin-like peptides and the interaction with their respective receptors have been elucidated to aid future treatment and imaging of epithelial cell-derived tumors. The presence of Bn receptors on tumor tissues is receiving amplified attention, both for tumor imaging as well as to target cytotoxic agents by using either radiolabeled Bn analogs or other cytotoxic agents formed by coupling various Bn receptor ligands using various linkers to various cytotoxic agents (Okarvi 2004; Zhou et al. 2004; Schroeder et al. 2010; Tweedle 2009; Okarvi 2008; Gonzalez et al. 2008) (Table 2). This receptor-mediated targeting approach is being used with many peptides (Okarvi 2004; Gonzalez et al. 2008; Okarvi 2008; Tweedle 2009; Schroeder et al. 2010). There is particularly interest in this receptor family for a number of reasons. Bn receptors are overexpressed in various major human cancers such as prostate, breast, and small cell lung cancer (Nanda et al. 2010). The Bn family of receptors, particularly GRPR, has been shown to be one of most overexpressed or ectopically expressed family of G protein-coupled receptors by small lung cancer cells (GRPR: 85–100%, NMBR: 55%, BRS: 3–25%), non-small cell lung cancer (GRPR: 74–78%, NMBR: 67%, BRS: 3–8%), pancreatic pancreatic cancer (GRPR: 75%, NMBR: 100%), prostate cancer (GRPR: 60–100%, 0%-NMBR, BRS-3), head and neck squamous cell cancers (GRPR: 100%), glioblastomas (GRPR: 85%), neuroblastomas (GRPR: 72%, NMBR: 46%, 0%, BRS: 3), breast cancer (GRPR: 40–70%, NMBR: 0%, BRS: 3), intestinal carcinoids (NMBR: 46%, 0%, GRPR, BRS: 3), and bronchial carcinoids (35%-BRS: 3, 4%-NMBR, GRPR: 0%) (Anderson and Welch 1999; Reubi et al. 2002; Moody et al. 2003; Jensen and Moody 2006; Sancho et al. 2011). These data illustrate that each Bn receptor subtype may be overexpressed on different types of tumors, and the same type of tumor may coexpress more than one Bn receptor subtype, indicating that universal bombesin analogs have the potential (Weiner and Thakur 2002) to visualize these tumors with high incidence. Mantey et al. (1997) and Pradhan et al. (1998) developed a common ligand, [DTyrP6P, βAla11P, PheP13P, NleP14P]Bn (Reubi 1995; Heasley 2001; Signore et al. 2001; Weiner and Thakur 2002; Laskin and Sandler 2004; Okarvi 2004) having high affinity to all receptor subtypes. A novel GRP variant, GRP1–46, isolated from pregnant ovine endometrium, was characterized and shown to have low affinity for both GRP-R and NMB-R in rodent and human cell lines and mobilized inositol phosphate in cell lines expressing GRP-R and NMB-R but not BRS-3 (Giraud et al. 2010). Owing to frequent aberrant expression in epithelial cancers, GRP-R has been explored for receptor-mediated targeting of imaging and therapeutic agents in human malignancies (Nanda et al. 2010).
Table 2
Bn analogs used in various imaging studies
SN | Bombesin analog | Amino acid position relative to Bn sequence in bold | References | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |||
1 | Bombesin | Pyr | Gln | Arg | Leu | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | |
2 | GRP (13–27) | Tyr | Pro | Arg | Leu | Gly | Asn | His | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | Breeman et al. (1999) |
3 | Litorin [pGlu6,Phe13]Bn(6–14) | _ | _ | _ | _ | _ | pGlu | Gln | Trp | Ala | Val | Gly | His | Phe | Met-NH2 | Durkan et al. (2007) |
4 | (a) Demobesin 3 [N40,Pro1,Tyr4]Bn, (b) Demobesin 4 [N40,Pro1,Tyr4,Nle14]Bn | N 4 -Pro | Gln | Arg | Tyr | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | (a) Met-NH2, (b) Nle-NH2 | Nock et al. (2005) |
5 | [Lys3]Bn | Pyr | Gln | Lys | Leu | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | |
6 | (a) [Tyr4]Bn, (b) [εLys3,Tyr4]Bn | Pyr | Gln | (a) Arg, (b) εLys | Tyr | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | |
7 | [Gln1,Tyr4]Bn | Gln | Gln | Arg | Tyr | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | Achilefu et al. (2002) |
8 | [Gly1]Bn | Gly | Gln | Arg | Leu | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | |
9 | (a) [Pro1,Tyr4]Bn (MP2346), (b) [Pro1,Tyr4,Nle14]Bn | Pro | Gln | Arg | Tyr | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | (a) Met-NH2, (b) Nle-NH2 | |
10 | [Cys0,Aca1]Bn(2–14) | Cys-Aca | Gln | Arg | Leu | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | |
11 | Bn(2–14) | _ | Gln | Arg | Leu | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | |
12 | (a) [Lys14]Bn(2–14), (b) [Lys3,Tyr4]Bn(2–14) | _ | Gln | Arg | (a) Leu, (b) Tyr | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | (a) Lys-NH2 (b) Met-NH2 | |
13 | [mIP]Bn(2–14) | mIP | Gln | Arg | Leu | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | Rogers et al. (1997) |
14 | Bn(4–14) | _ | _ | _ | Leu | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | Abiraj et al. (2008) |
15 | [Ser4,5,6]Bn(4–14) | _ | _ | _ | Ser | Ser | Ser | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | |
16 | [ACMpip5,Tha6,βAla11,Tha13,Nle14]Bn(5–14) (MP2653) | _ | _ | _ | _ | AC Mpip | Tha | Gln | Trp | Ala | Val | βAla | His | Tha | Met-NH2 | |
17 | (a) [Tyr5,DPhe6,ψPhe14] Bn(5–14), (b) [DPhe6,Leu13ψPhe14] Bn(6–14), a (c) [DPhe6,LeuNHEt13,des-Met14] Bn(6–14) | _ | _ | _ | _ | Tyr | D-Phe | Gln | Trp | Ala | Val | Gly | His | (a, b) Leu-ψ, (c) Leu-NHEt | (a, b) Phe-NH2 (c) _ | |
18 | [DTyr6,βAla11,Thi13,Nle14] Bn(6–14) | _ | _ | _ | _ | _ | D-Tyr | Gln | Trp | Ala | Val | Ala | His | Tha | Met-NH2 | |
19 | Demobesin 1 [N401,bzdig0,DPhe6,Leu-NHEt13,des-Met14]Bn(6–13) | _ | _ | _ | _ | N 4 -bzdig | D-Phe | Gln | Trp | Ala | Val | Gly | His | Leu-NHEt | _ | |
20 | RM1 [N40DPhe6,Sta13,Leu14] Bn(6–14) | _ | _ | _ | _ | _ | N 4 -DPhe | Gln | Trp | Ala | Val | Gly | His | Sta | Leu-NH2 | |
21 | [Lys6]Bn(6–14) | _ | _ | _ | _ | _ | Lys | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | Achilefu et al. (2002) |
22 | Bn(7–14) | _ | _ | _ | _ | _ | _ | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | Van de Wiele et al. (2000), van de et al. (2001), Achilefu et al. (2002), Hu et al. (2002), La Bella et al. (2002), Hoffman et al. (2003), Smith et al. (2003a, b, c), Okarvi (2004), Rogers et al. (2004), Scopinaro et al. (2004), Wild et al. (2005), Alves et al. (2006), Yang et al. (2006), Zhang et al. (2006), Garcia et al. (2007a, b), Kunstler et al. (2007), Ma et al. (2007), Waser et al. (2007), Garrison et al. (2008), Lane et al. (2008), Gourni et al. (2009), Liu et al. (2009a, b), Mansi et al. (2009), Paterson et al. (2010), Retzloff et al. (2010), Schroeder et al. (2010) |
23 | (a) Demobesin 5 [(N4Bzdig)0]Bn(7–14), (b) Demobesin 6 [(N4Bzdig)0,Nle14] Bn(7–14) | _ | _ | _ | _ | _ | N 4 -Bzdig | Gln | Trp | Ala | Val | Gly | His | Leu | (a) Met-NH2, (b) Nle-NH2 | |
24 | (a) [Cha13,Nle14]Bn(7–14), (b) [Cha13]Bn(7–14) | _ | _ | _ | _ | _ | _ | Gln | Trp | Ala | Val | Gly | His | Cha | (a) Nle-NH2 (b) Met-NH2 | |
25 | [Nle14]Bn(7–14) | _ | _ | _ | _ | _ | _ | Gln | Trp | Ala | Val | Gly | His | Leu | Nle-NH2 | |
26 | (a) [NMeGly11,Sta13,Leu14] Bn(7–14), (b) [FA0101013,Leu14]Bn(7–14) | _ | _ | _ | _ | _ | _ | Gln | Trp | Ala | Val | NMe Gly | His | (a) Sta, (b) FA01010 | Leu-NH2 | |
27 | [βAla11,Phe13,Nle14]Bn(7–14) | _ | _ | _ | _ | _ | _ | Gln | Trp | Ala | Val | βAla | His | Phe | Nle-NH2 | de Visser et al. (2007) |
28 | [His(3Me)11,Sta13,Leu14]Bn (7–14) | _ | _ | _ | _ | _ | _ | Gln | Trp | Ala | Val | His(3Me) | His | Sta | Leu-NH2 | Hohne et al. (2008) |
29 | [des-Met14] Bn(7–14)NH2 | _ | _ | _ | _ | _ | _ | Gln | Trp | Ala | Val | Gly | His | Leu-NH2 | _ | Rogers et al. (1997) |
30 | [DTyr6,des-Met14] Bn(6–13)NHEt | _ | _ | _ | _ | _ | D-Tyr | Gln | Trp | Ala | Val | Gly | His | Leu-NHEt | _ | Breeman et al. (1999) |
31 | [Tyr5,DPhe6] Bn(5–13)NHEt | _ | _ | _ | _ | Tyr | D-Phe | Gln | Trp | Ala | Val | Gly | His | Leu-NHEt | _ | Maina et al. (2006) |
32 | RC-3095 [D-Tpi6,Leu13, ψLeu14]Bn(6–14) | _ | _ | _ | _ | _ | D-Tpi | Gln | Trp | Ala | Val | Gly | His | Leuψb | Leu-NH2 | Varvarigou et al. (2002) |
33 | Pensin | _ | _ | _ | _ | _ | dPEG 4 | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | |
34 | AMBA | _ | _ | _ | _ | _ | CH2CO-Gly-4 amino benzyl | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | |
On the other hand, for somatostatin receptors, receptor-mediated imaging has been shown to be safe and clinically useful and is now being widely used in clinical practice (Forrer et al. 2007; Van Essen et al. 2009). In the case of neuroendocrine tumors (carcinoids, pancreatic endocrine tumors), in most studies the majority (>80%) overexpress or ectopically express one or more of the five classes of G protein-coupled somatostatin receptors (sst1–5), usually the sst2 subtype (Krenning et al. 1993; Jensen 1997; Gibril and Jensen 2004; Forrer et al. 2007; Van Essen et al. 2009), in an analogous fashion to the tumors listed above, overexpressing one of the Bn receptor family. The use of 111In-penetreotide for somatostatin receptor scintigraphy (SRS) is now a standard clinical method to image these tumors (Kwekkeboom et al. 2000; Gibril and Jensen 2004; Metz and Jensen 2008). Unfortunately, many of the common lethal tumors do not overexpress somatostatin receptors, as occurs in the neuroendocrine tumors. Therefore, if receptor-mediated imaging is going to be used for these tumors, some other family of receptors needs to be considered. The Bn family of receptors could fulfill this requirement for a number of these tumors (Zhou et al. 2004; Okarvi 2004, 2008; Gonzalez et al. 2008; Tweedle 2009; Schroeder et al. 2010).
BLPs also function as potent growth factors, sometimes in an autocrine fashion, for many common malignant tumors including those of lung, pancreas, head and neck, CNS (glioblastomas), kidney, prostate, breast, colon/rectum, ovary, and stomach (Preston et al. 1996; Moody et al. 2003; Yegen 2003; Jensen and Moody 2006). This raises the possibility that receptor antagonists of Bn receptors may have cytotoxic effects for a number of these tumors, and the possibility that targeting Bn receptors on these tumor cells may have additional cytotoxic effects by interrupting this autocrine stimulatory effect. There are no effective nonpeptide antagonists or agonists for GRPR or NMBR, which are primarily overexpressed by tumors. The pharmacology of these receptors has been well studied, especially in nonhuman cells. Both selective agonists and at least eleven chemical classes of antagonists, with varying degrees of selectivity, have been described (Battey et al. 1992; Jensen et al. 2008; Gonzalez et al. 2009). Therefore, pharmacologically, both agonists and antagonists exist that can be used for Bn receptor targeting strategies.
3.2 Gallium Radiometal
3.2.1 Radionuclide of Choice
To date, a wide variety of radionuclides (e.g., 99mTc, 188Re, 90Y, 111In, 18F, 64Cu, 177Lu, 149Pm, and 68Ga) have been used in the development of BB2-targeting peptides (Heppler Froidevaux Eberle et al. 2000; Hu et al. 2002; Hoffman et al. 2003; Smith et al. 2003a, c, d; Zhang et al. 2004, 2006, 2007; Garrison et al. 2007) (Table 2). The selection of the radionuclide for the GPR-R-targeted Bn analog is largely influenced by the desired function of the radiopharmaceutical. Diagnostic nuclear imaging agents that utilize radionuclides with gamma emission are suitable for single-photon emission computed tomography (SPECT), and those with positron emission can be used for positron emission tomography (PET). Radionuclides are chosen to deliver a maximum radiation dose to the cancerous tissue for targeted radiotherapy, at the same time attempting to ensure minimal damage to nontarget tissues (Volkert and Hoffman 1999).
An important aspect for wide use of 68Ga in clinical PET is its chemical form and concentration after elution from the generator. There is concern about 68Ge breakthrough and contamination of the generator column material. Gallium is a short-lived positron emitter and consists of two naturally occurring isotopes: 69Ga (60.1% natural abundance) and 71Ga (39.9% natural abundance). Three radioisotopes can be produced for labeling of radiopharmaceuticals. Two of these, 66Ga (t 1/2 = 9.5 h) and 68Ga (t 1/2 = 68 min), decay by β+ emission and are therefore suitable for PET imaging; 67Ga (t 1/2 = 8 h) decays by γ emission and is used for SPECT imaging. Its production does not require a cyclotron since it is available as a generator nuclide. Different 68Ge-carrier column materials have been proposed and used in the past, among them inorganic oxides such as Al2O3, TiO2, or SnO2. Recently, a TiO2-based generator has become commercially available, being eluted with 0.1 mol/L HCl. Extraction of 68Ga eluate from a 68Ge/68Ga generator has been reported (Meyer et al. 2005). Briefly, final concentration of 5.5 M HCl is used for extracting 68Ga, which can be adsorbed on a strong anion exchanger as anionic chlorocomplexes of 68Ga(III). Gallium adsorbed on resin is flushed with a stream of N2 and then eluted with H2O in small volumes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree