Fig. 1
HPLC profiles of DOTA-mAbs conjugations. (Right) reaction mixture. (Left) purified conjugate
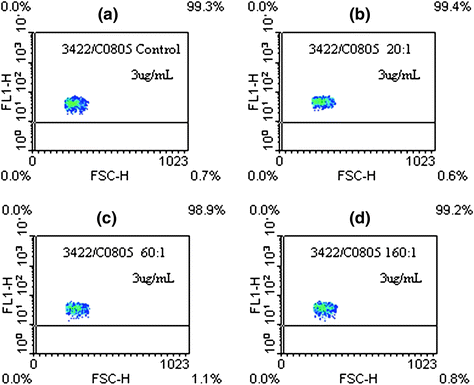
Fig. 2
Flow cytometric analysis. a Unmodified nimotuzumab, b (DOTA-Bz-SCN)4-5-h-R3, c (DOTA-Bz-SCN)8-9-h-R3, d (DOTA-Bz-SCN)14-15-h-R3
Several groups have reported radiolabeling of mAbs with 90Y and 177Lu using acyclic and cyclic ligands (Kukis et al. 1998; Motta-Hennessy et al. 1991; Blend et al. 2003; Griffiths et al. 2003; Coliva et al. 2005; Mohsin et al. 2007; Papi et al. 2010). 90Y and 177Lu were selected due to their appropriate half-lives for labeling mAbs, well-known chelation chemistry, residualization after internalizing mAbs, and reasonable availability (Liu 2008; Nayak and Brechbiel 2009). Since 90Y and 177Lu are used for endoradiotherapeutic purposes, this study was undertaken to obtain radioimmunoconjugates with high specific activities. Radioimmunoconjugates with high chemical and radiochemical purity (>98%) and specific activity up to 2 GBq/mg (Fig. 3) were obtained. The quality of the radionuclide and the volume of radiolabeling mixture influenced the radiolabeling efficiency (Vera et al. 2011). The radiolabeling yields were higher and more reproducible for radioimmunoconjugates formed with p-SCN-Bn-DOTA. Our results are similar to or higher than those previously reported (Xiques et al. 2009; Blend et al. 2003; Coliva et al. 2005; Mohsin et al. 2007; Papi et al. 2010; Liu et al. 2010; Beckford Vera et al. 2007; Lee et al. 2005; Zacchetti et al. 2009). The radiolabeling yields and specific activities achieved are suitable for radiopharmaceutical preparation. It seems to be that the radioactive concentration, not only in the radionuclide preparation but also in the final reaction mixture, plays an important role in the labeling of mAbs with radiometals.
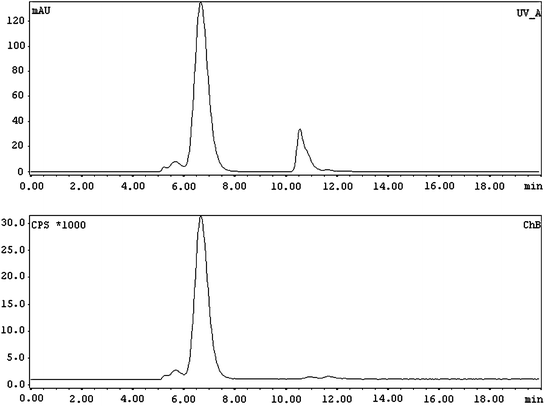

Fig. 3
Typical radioHPLC profile of the 177Lu/90Y-DOTA-(nimotuzumab/trastuzumab). (Upper panel) UV profile in which the main peak (left) and the second peak (right) correspond to the radioimmunoconjugate DTPA added for quality control, respectively. (Lower panel) Radioactive profile; almost all the activity is associated with the mAb
3.2 In Vitro Studies
Radioimmunoconjugates without significant loss of biological activity were obtained. The binding of the radioimmunoconjugate to the cell lines was receptor specific as estimated by the ability to compete with native unmodified mAbs (Vera et al. 2011; Beckford Vera et al. 2011). The immunoreactivity fraction of labeled mAbs was higher than 90%. The results showed that the conjugation and radiolabeling did not significantly affect the immunoreactivity, specificity, and affinity of the conjugate.
The results from the cell proliferation assay with 90Y-mAbs conjugates, unmodified mAbs, and 90Y showed that 90Y-mAbs conjugates increased cell growth proliferation compared with unmodified mAbs and 90Y (Fig. 4). The cell proliferation study showed that 90Y-DOTA-Bn-h-R3 conjugates, such as nimotuzumab, selectively bound to cells with moderate to high EGFR expression due to the necessity for bivalent binding in order to have stable attachment to the cell surface (Garrido et al. 2011). The increased cell proliferation inhibition of 90Y-DOTA-Bn conjugates over unmodified mAbs or 90Y alone in treated cells might be explained due to the crossfire effect of the 90Y radiation and its residualization.
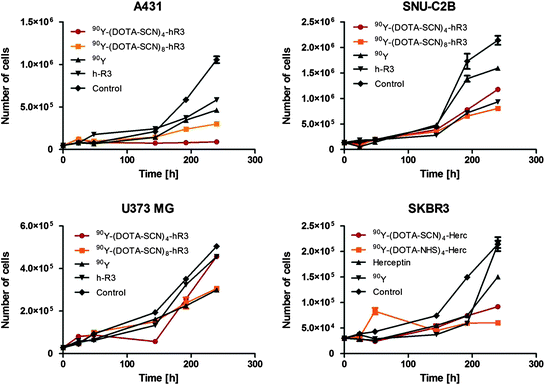

Fig. 4
Cell growth inhibition assay
In a previous study (Costantini et al. 2007), trastuzumab labeled with 111In showed 1.2-fold higher toxicity in cultured SKBR3 cells than unlabeled trastuzumab, whereas in a recent study (Rasaneh and Rajabi 2010), trastuzumab labeled with 177Lu showed 4.5-fold higher toxicity in cultured SKBR3 cells than unlabeled trastuzumab. In this study, it was observed that 90Y-(DOTA-NHS)8-9-Herceptin and 90Y-(DOTA-Bn)8-9-Herceptin showed 3.0- and 1.7-fold higher toxicity, respectively, than unlabeled trastuzumab.
Consequently, targeted RIT with 90Y-(h-R3 or trastuzumab) could be a promising approach to increase the apoptotic effect of h-R3 and trastuzumab in cells with intermediate to high EGFR expression. Due to the energy and maximal tissue penetration of the beta particles emitted by 90Y and the results of the cytotoxicity studies, 90Y-h-R3/Herc might be suitable for treatment of large-diameter solid tumors.
3.3 In Vivo Studies
Uptake of the 177Lu-DOTA-Bn-h-R3 conjugate in healthy animals behaves similarly to mAbs labeled with radiolanthanides through macrocyclic ligands (Mohsin et al. 2006, 2007). It is well known that monoclonal antibodies are cleared through the reticuloendothelial organs, so spleen and liver uptakes should be commonly observed (Mohsin et al. 2006, 2007). The study revealed that the number of chelators bound to the monoclonal antibody influenced blood clearance and liver uptake. Liver uptake was significantly higher for the 177Lu-DOTA-Bn-h-R3 conjugate with the higher DOTA/h-R3 ratio (p < 0.01). These results are in agreement with those reported by Knogler et al. (2006). The uptakes in liver, spleen, and bones observed in our study were lower than those reported by Mohsin et al. (2007) for conjugates modified with 4–5 groups of p-SCN-Bn-DOTA or p-SCN-Bn-DTPA. The release of the radiometal from the conjugate and its metabolism produces nonspecific accumulation of the radioactivity in different tissues and gives us an idea of the stability of the conjugate. Tumor-to-blood ratio calculations are useful for this estimation. The liver-to-blood ratio slightly increased over time for 177Lu-(DTPA-Bn)4-5-h-R3 and 177Lu-(DOTA-Bn)8-9-h-R3 but for 177Lu-(DOTA-Bn)4-5-h-R3, indicating its relatively higher stability compared with the first two mentioned conjugates. The bone-to-blood ratio did not increase with time for all radioimmunoconjugates, suggesting high stability of the metal–ligand complexes.
DOTA derivatives were chosen over DTPA derivatives for labeling the mAbs with 90Y and 177Lu based on the cumulative published data where it is stated that macrocyclic ligands form highly stable complexes with yttrium for in vivo applications (Kukis et al. 1998; Liu et al. 2003; Liu and Edwards 2001b). However, even when those ligands are commercially available, they have the disadvantage of the several parameters which can affect the radiolabeling (Liu 2008). The results of the biodistribution study of 177Lu-DOTA-Bn-h-R3 in mice with SNU-C2B tumor xenograft, previously reported (Vera et al. 2011), showed significant uptake in blood, liver, kidneys, tumor, and skin connected to tumor. The maximum and average uptake of radioimmunoconjugate for SNU-C2B tumor was 10.0 ± 1.9% ID/g. In this study (Vera et al. 2011), tumor uptake of 177Lu-DOTA-Bn-h-R3 was higher than reported by Alejo Morales-Morales et al. (1999), Velikyan et al. (2005), and Lee et al. (2009), and similar to that reported by Milenic et al. (2002).
Biodistribution studies of 177Lu-(DOTA-Bn) n -h-R3 (n = 4–5) were conducted in A431 human epidermal carcinoma xenografts in BALB/c nude mice. Tumor uptake reached a maximum value of 22.4 ± 3.1% ID/g at 72 h. From 72 to 216 h the average tumor uptake was 18.3 ± 0.3% ID/g. Bone uptake was low, with maximum value of 2.7 ± 1.1% ID/g at 24 h and an average of 1.6 ± 0.5% ID/g from 72 to 264 h. Tumor-to-nontumor tissue ratios reached maximum values of 2.6, 2.4, 6.1, 6.6, 23.5, and 17.5 for blood, liver, spleen, kidneys, muscle, and bone, respectively. When 177Lu-(DOTA-Bn) n -h-R3 (n = 4–5) conjugate was applied locoregionally to A431 xenografts in BALB/c nude mice, tumor uptake was significantly higher at 24 and 72 h than observed for intravenous application (p < 0.001). Tumor uptake peaked at 32.7 ± 2.9% ID/g at 72 h. Locoregionally application of the 177Lu-DOTA-Bn-h-R3 conjugate showed T/nT ratios 4.6, 9.8, 10.5, 9.8, and 11.7 times higher for blood, liver, spleen, lungs, and kidneys, respectively, than those calculated for IV application. At 72 h, the T/nT ratios mentioned above were two times higher for locoregional (LR) than IV application. In this study, the tumor uptake values and tumor-to-nontumor ratios are higher than those previously reported for h-R3 and the parental murine mAb labeled with 99mTc and 90Y (Almqvist et al. 2006; Tabrizi et al. 2006; Morales et al. 2000).
Dosimetric calculations showed that the 177Lu-DOTA-h-R3 radioimmunoconjugate will deliver the highest dose to the tumor. The smaller the tumor, the higher the dose deposited. The results obtained are in agreement with those previously reported for similar 177Lu conjugates (Brouwers et al. 2004; Zacchetti et al. 2009). The results from extrapolation of animal data to humans in this study showed doses to normal organs lower than those reported by Iznaga-Escobar et al. (1998) for 99mTc-h-R3. It is necessary to mention that, in the extrapolation of the animal data obtained in this study to humans, cross-reactivity of nimotuzumab is not taken into account. In this respect locoregional anti-EGFR RIT might be a more promising option.
4 Conclusion
Radioimmunoconjugates with high radiolabeling yield, radiochemical purity, and specific activity were obtained without significant loss in biological activity. Cytotoxicity studies showed the potential of the radioimmunoconjugates over unmodified monoclonal antibodies to inhibit cell proliferation in cell lines with intermediate to high EGFR/HER2 expression. 177Lu-DOTA-h-R3 may have potential for further evaluation as a radiopharmaceutical for RIT of EGFR-overexpressing tumors.
Acknowledgments
We gratefully acknowledge Isotope Technologies Garching GmbH (ITG), Munich, Germany for providing the 177Lu n.c.a. The authors would like to thank Dr. Normando Iznaga and Dr. Angel Casacó (Center of Molecular Immunology) for their outstanding support in the flow cytometric study.
References
Beckford Vera DR, Eigner S, Beran M, Henke KE, Laznickova A, Laznicek M et al (2011) Preclinical evaluation of (177)lu-nimotuzumab: a potential tool for radioimmunotherapy of epidermal growth factor receptor-overexpressing tumors. Cancer Biother Radiopharm 26(3):287–297CrossRef
Beckford Vera DR, Eigner S, Henke KE, Lebeda O, Melichar F, Beran M (2011) Preparation and preclinical evaluation of 177Lu-nimotuzumab targeting epidermal growth factor receptor overexpressing tumors. Nucl Med Biol 39(1):3–13CrossRef
Beckford Vera DR, Xiques Castillo A, Leyva Montaña R, Pérez Malo M, Gonzalez Casanova E, Zamora Barrabi M (2007) New radioimmunoconjugate 90Y-DOTA-h-R3. Synthesis and radiolabeling. Nucleus 41:3–8
Blend MJ, Stastny JJ, Swanson SM, Brechbiel MW (2003) Labeling anti-HER2/neu monoclonal antibodies with 111In and 90Y using a bifunctional DTPA chelating agent. Cancer Biother Radiopharm 18(3):355–363PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree