Fig. 1
CT-guided core-needle biopsy of the mediastinal tumor showed a moderately differentiated cellular neoplasm with syncytial growth pattern lacking mitotic activity. Immunohistochemically the tumor was positive for EMA (Fig. 1) while lacking immunoreactivity for cytokeratin, S100, chromogranin A, or synaptophysin. Intraoperative smear preparation of the spinal part of the tumor showed neoplastic cells that were partially arranged in whorls and displayed indistinct cell borders and monotonous round to oval nuclei with finely granular chromation and occasional intranuclear pseudoinclusions (Fig. 2). On hematoxylin-eosin-stained paraffin sections, occasional mitotic figures and rare foci of necrosis were identified (Fig. 3). Both histologic tumor type and immunohistochemical profile corresponded to that of the mediastinal tumor. Proliferation index as assessed by immunohistochemistry for MIB-1 (Ki67) reached up to 3–5%. Based upon these findings, a diagnosis of meningotheliomatous meningioma was issued. It was commented that, although the tumor fell short of formal criteria of atypical meningioma (WHO grade I), the borderline increased mitotic and proliferative activity as well as the focal presence of necrosis possibly indicated increased risk of progression or recurrence, and close follow-up was suggested
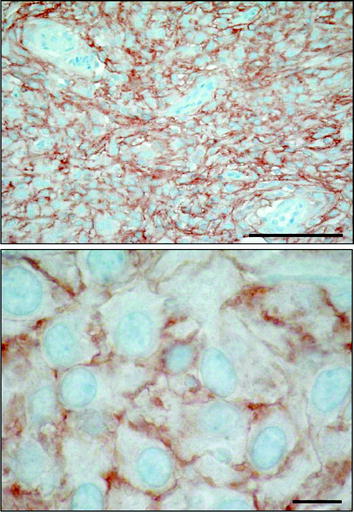
Fig. 2
SSTR type 2 immunohistochemical staining showing strong positivity, preferably membranous, in the majority of tumor cells. Bars = 0.1 mm (above) and 0.01 mm (below)
1.3 Pretherapeutic Imaging
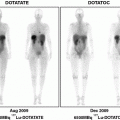
CT scan of the thorax and MRI scan of the spine revealed a large prevertebral tumor, which extended to the spinal canal. This tumor caused compression of the spinal cord at levels C7 to D4, leading to myelopathy with hyperintense signal alteration on T2-weighted MRI images (Figs. 3, 4, 5). CT scan of the skull revealed, in addition to the spinal meningioma, several cranial meningiomas with typical contrast enhancement, partially with calcification. To verify the histological SSTR expression and evaluate the possibility of peptide receptor radionuclide therapy (PRRNT) with 90Y-DOTA-TOC, somatostatin receptor SPECT/CT was done (Fig. 6). The somatostatin receptor SPECT/CT with 111In-DTPA-d-Phe-1-octreotide detected a somatostatin receptor-positive mediastinal tumor with infiltration of multiple vertebrae, dura, and intervertebral foramina C7–D4, partially with Krenning score >2 (1 = lower than liver uptake; 2 = equivalent to liver uptake; 3 = higher than liver uptake). No other SSTR-positive lesions were visible; especially, the intracranial lesions showed no SSTR expression.
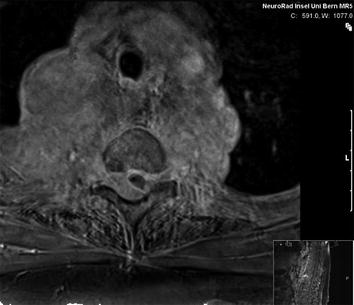
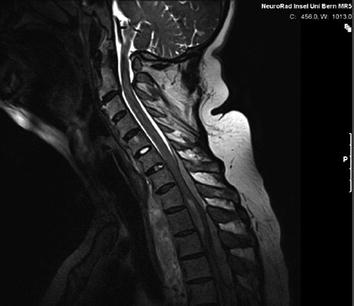
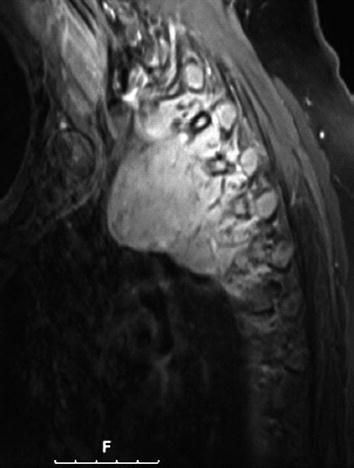
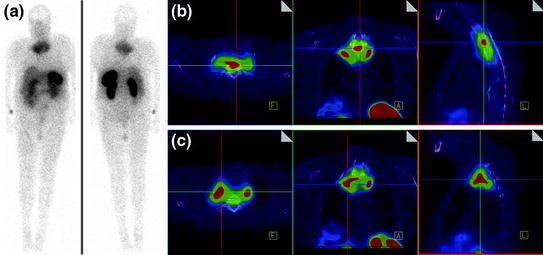

Fig. 3
MRI of the spine: large intraspinal tumor, which extended to the mediastinum. MRI: T1 GRE (FLASH) with contrast

Fig. 4
MRI of the spine: large intraspinal tumor, which extended to the mediastinum. MRI: T2* GRE (FLASH)

Fig. 5
MRI of the spine: large intraspinal tumor, which extended to the mediastinum. MRI: T1 TSE-fs with contrast

Fig. 6
Somatostatin receptor SPECT/CT and whole-body scan with 111In-DTPA-d-Phe-1-octreotide: somatostatin receptor-positive mediastinal tumor with infiltration of multiple vertebrae, dura, and intervertebral foramina C7–D4, partially with Krenning score >2
1.4 Therapy
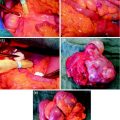
C7 to D4 laminoplasty was performed, and the intraspinal, extradural part of the well-vascularized tumor was microsurgically removed using a diode-pumped solid-state (DPSS) laser system (RevoLix). Small amounts of tumor located ventral to the dural sac and in the neural foramina were not amenable to surgical resection. Intraoperative ultrasound demonstrated no residual compression of the spinal cord. Combined motor evoked potential (MEP) and somatosensory evoked potential (SEP) neuromonitoring during spine surgery detected no significant intraoperative MEP/SEP changes, and no new postoperative neurological deficits were noted. Due to secondary bleeding with compression of the myelon and worsening of the neurological deficits, reoperation to evacuate hematoma had to be done in an emergency situation. No other postoperative complications occurred (Figs. 7, 8, 9).
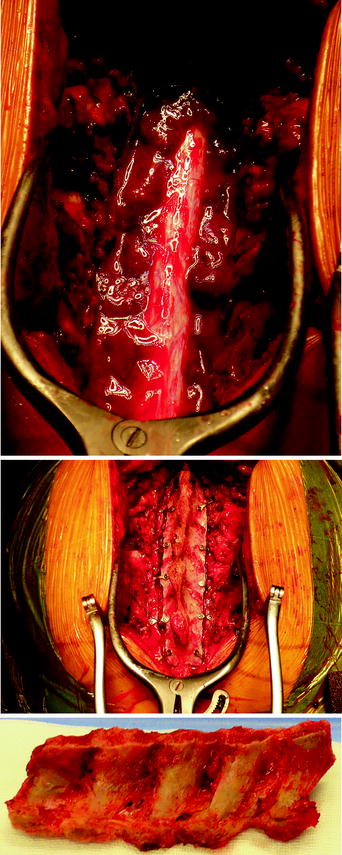

Figs. 7–9
C7 to D4 laminoplasty was performed, and the intraspinal, extradural part of the well-vascularized tumor was microsurgically removed using a diode-pumped solid-state (DPSS) laser system
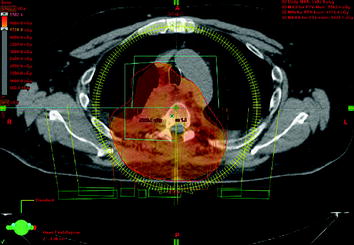
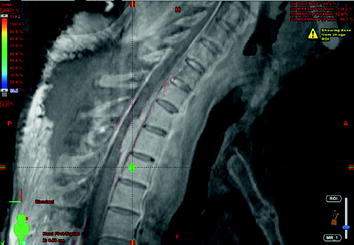
Postoperative fractionated stereotactic radiation therapy using the volumetric modulated arc therapy (VMAT) technique (RapidArc) was started to prevent further paraparesis by tumor progression. 6D image guidance was done with the ExacTrac X-ray system. The residual intraspinal and intraforaminal parts of the tumor received up to 51 Gy, and the mediastinal mass up to 55 Gy. The spinal cord (organ at risk) received less than 48 Gy. For the lung (organ at risk), V20 Gy was 25% and the mean lung dose was 18.6 Gy (Figs. 10, 11, 12, 13).
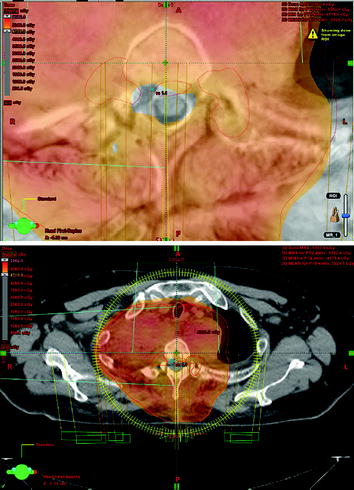

Figs. 10–11
Outer red line: planning target volume receiving up to 55 Gy; inner red lines: SSTR-positive intraspinal lesions with Krenning score >2, receiving up to 51 Gy; the myelon received less than 48 Gy, possible with VMAT technique

Fig. 12
Red line: planning target volume receiving up to 55 Gy; the myelon received less than 48 Gy, possible with VMAT technique

Fig. 13
Image fusion of sagittal MRI with sagittal CT for radiation treatment planning (red line: intraspinal gross tumor volume with intense somatostatin receptor expression, Krenning score >2)
2 Discussion
Primary tumors of the spinal cord are uncommon; only about 5% are primary intramedullary tumors or arise from the spinal meninges. The most common tumor types are meningioma, ependymoma, and schwannoma (CBTRUS 2000; Mawrin and Perry 2010; Perry et al. 1999; Nakasu et al. 2009; Lee et al. 2010; Engelhard et al. 2010). Our case is even more exceptional because the meningioma grew out of the spinal canal through the intervertebral foramina and so presented with a manifestation in the spinal canal and in the posterior mediastinum as well. Only a few cases similar to ours have been reported (Buchfelder et al. 2001; Barbanera et al. 2007; Incarbone et al. 2008). Most of the authors of these case reports recommend surgical therapy, sometimes with a two-stage approach (Mimoto et al. 1991; Yoon et al. 2007; Goldsmith et al. 1994; Jenkinson et al. 2006; Gambardella et al. 2003).
Meningiomas are generally slowly growing and produce neurological signs and symptoms by compression on adjacent structures. The classic and common variant is the meningothelial meningioma. It is known that the clinical course is often difficult to predict; even histopathologically benign meningiomas (WHO grade I) can recur despite seemingly complete surgical resection. The major clinical factors in recurrence are extent of resection and the grading. However, at least for WHO grade I cranial meningioma, no significant influence of extent of resection (Simpson grade I-IV) on tumor recurrence has been found (Sughrue et al. 2010). Atypical (WHO grade II) and anaplastic meningioma (WHO grade III) have a higher recurrence rate than benign meningioma. Levy et al. (1982) holds that the presence of an extradural component in spinal meningiomas is associated with more aggressive behavior and higher recurrence rate. Solero et al. (1989), analyzing a series of 174 patients operated on for spinal meningiomas, drew the conclusion that extradural component is not associated with higher incidence of recurrence. None of the aspects used in the WHO grading to define malignancy in meningiomas (hypercellularity, loss of architecture, nuclear pleomorphism, high mitotic index, focal necrosis, and brain invasion) were present in our reported case. However, MR imaging and somatostatin receptor imaging showed infiltration of multiple vertebrae, a criterion for malignancy and aggressiveness.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree