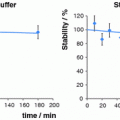
Fig. 1
Successive steps of post-processing of 68Ge/68Ga radionuclide generator eluates via cation exchangers (Zhernosekov et al. 2007; Asti et al. 2008). a 68Ga is adsorbed online by passing the 68Ge/68Ga radionuclide generator eluate through a 53 mg cation exchange (CEX) AG 50 Wx8 cartridge. Chemical impurities such as FeIII, ZnII, TiIV, but also a significant part of the breakthrough 68Ge are eluted almost completely from the cation exchange column into waste. b In an additional washing step using 1 mL of 0.15 N HCl/acetone mixture (solution N1), remaining FeIII, ZnII, TiIV, and 68Ge are removed from the cation exchange cartridge, while 68Ga efficiently stays in the resin. c Transfer of purified 68Ga from the CEX resin into a (labeling) vial by applying 400 μL of 0.05 N HCl/98% acetone (solution N2). Overall, 97 ± 2% of the 68Ga initially obtained from the generator is isolated even by 3 min after the elution procedure
In an additional washing step using 1 mL of HCl/acetone mixture (solution N1), remaining impurities are removed from the cation exchange cartridge, while 68Ga efficiently stays in the resin (Fig. 1b).
Finally, the transfer of purified 68Ga from the resin into a (labeling) vial is almost quantitative. Applying 400 μL of 0.05 N HCl/98% acetone (solution N2), overall, 97 ± 2% of the 68Ga initially obtained from the generator is isolated by even 3 min after the elution procedure (Fig. 1c).
This 68Ga is chemically pure. The 68Ge content/breakthrough is decreased from >10−3 initially to <10−7% finally. The final volume of the purified 68Ga fraction is low, as is the pH (400 μL 0.05 N HCl/acetone). As the purified 68Ga is eluted directly into a labeling vial containing amounts of precursor, the removal of metallic impurities results in high labeling yields and 68Ga radiopharmaceuticals of high specific activities. For, e.g., DOTATOC, labeling is achieved in pure water (adding 5 mL ultrapure water to the precursor creates a final pH of 2.3 after adding the 68Ga fraction of 400 μL 0.05 N HCl/acetone), thus avoiding any addition of buffers. About >95% labeling at 10 min of >450 MBq/nmol DOTATOC is obtained. Overall, ca. 65% product yields are achieved (not corrected for decay) with respect to the initially eluted 68Ga.
3 Combined Cation and Anion Exchange-Based Post-Processing
The radionuclide 68Ga is desorbed from the resin quantitatively with 0.4 mL of 0.05 N HCl/98% acetone solution (N2). Contents of the acetone in the reaction mixture are low and nontoxic (Zhernosekov et al. 2007; Roesch 2010). They are easily reduced to nondetectable levels within labeling procedures at elevated temperature. They might be, however, avoided for direct in vivo applications or for radiolabeling reactions performed under high temperature or for chemical reactions requiring pure aqueous media exclusively.
The aim of present work is to develop an improved column-based chemical strategy combining anion exchange (AEX) processing. Direct preconcentration of 68Ga from the original eluate and its purification are supposed to be performed on the cation exchanger according to Zhernosekov et al. (2007). This 68Ga can be eluted with hydrochloric acid solutions of >2 N concentration. For effective transfer of the purified 68Ga into the aqueous phase of low acidity and small volume a secondary microcolumn, now AEX based, is introduced into the process, which allows direct readsorption of gallium eluted form the cation exchanger. From the AEX, 68Ga can be finally striped with a small volume of pure water. For this purpose a typical anion exchanger and a novel extraction chromatographic resin based on N,N,N’,N’-tetra-n-octyldiglycolamide (TODGA) is applied (Loktionova et al. 2011).
A conceptual flow sheet of the system, including the combination of cation exchange and anion exchange, e.g., AG 1 × 8 or TODGA, columns is presented in Fig. 2. The columns are connected consecutively via three-way valves. In the first step, 7 mL 0.1 N HCl passes through the generator and the cation exchange resin into the waste. Step 2 involves eluting the cation exchanger with 1 mL of hydrochloric acid/acetone solution N1 into the waste. The third step is the nearly quantitative transfer of 68Ga from the cation exchange column onto the anion exchanger resins with 3 mL of 5 or 4 M HCl. [This represents a significant improvement compared with other anion exchange-based procedures, as high concentrations of HCl and large volumes are avoided (Schumacher and Maier-Borst 1981; Meyer et al. 2004)]. While 68Ga is quantitatively adsorbed on both resins, the 3 mL of HCl continues to the waste vial, thereby removing the acetone introduced at the CEX step via solution N1. (Use of acetone may even be completely avoided by removing the high-performance purification of 68Ga from metallic impurities via solution N1. However, in view of the idea of almost completely removing 68Zn, as much Fe as possible, and in particular 68Ge, this special feature of the process should be retained.)
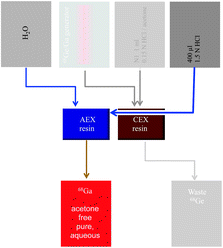

Fig. 2
Essentials of the combined cation (CEX) and anion exchanger (AEX)-based post-processing (Loktionova et al. 2011). The purified, solid-phase-adsorbed 68Ga is effectively transfered to subsequent anion exchange-based processing and eluted from it using, e.g., water
From the supplementary anion exchange, e.g., AG or TODGA columns, 68Ga is eluted using 0.3 or 1.0 mL water, respectively, into the product vial. With 98% effectivity for the cation exchange part, 92 and 98% yields were obtained for desorbing 68Ga from the anion exchange and chromatographic TODGA columns, respectively. The overall yield in the final 0.3–1.0 mL water fraction is 87 ± 5% (for AG 400 1-X8) and 96% (for TODGA resin) with respect to the initial generator eluate.
4 Post-Processing Towards Nonaqueous Systems for Labeling Lipophilic Compounds
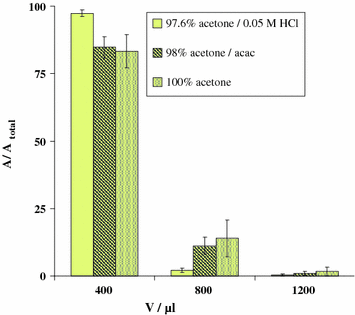
While almost all currently used 68Ga radiopharmaceuticals, particularly labeling precursors, are compounds that are easily soluble in aqueous solutions, some promising 68Ga tracers requiring nonaqueous synthesis conditions may be developed (Roesch and Riss 2010; Riss et al. 2010). The other direction for continuing development of the cation exchange post-processing thus proposes a convenient method for 68Ga-labeling under anhydrous conditions using solid-phase-derived gallium acetylacetonate, [68Ga]Ga(acac)3. Again, the initial aqueous generator eluate is first transferred online to a cation exchange resin (SCX in the present case) and 68Ga is absorbed quantitatively. From this resin, 68Ga is eluted with different acetone-based, nonaqueous solvent systems (Zoller et al. 2010). The resin is washed with solution N1 (0.15 N, 20% in acetone, 1 mL). The resin is dried in a stream of argon for 2 min, followed by elution of the trapped radioactivity as a [68Ga]Ga(acac)3 complex using 2% acetylacetone in acetone in volumes ranging from 200 to 1,600 μL. For comparison, the cation exchange resin was also eluted with 97.6% acetone/0.05 M HCl or 100% acetone in the same manner (Fig. 3). More than 95% of the generator-eluted 68Ga was obtained from the cation exchange resin with 600 μL of 98% acetone/2% acetylacetone mixture, providing n.c.a. [68Ga]Ga(acac)3 as labeling agent. The identity and purity of the labeling agent were verified via radio-thin-layer chromatography (TLC) using 1 μL sample aliquots on reverse-phase TLC sheets. Radio-TLC was developed in ethyl acetate:ethanol 1:1 (v/v) with [68Ga]Ga(acac)3 R f = 0.85 and 68Ga3+ R f = 0.0.