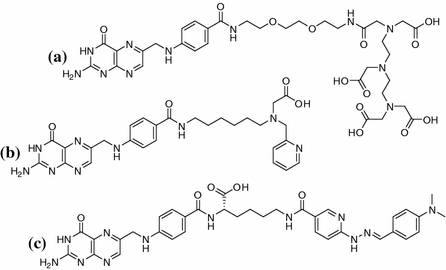
Fig. 1
Structure of folic acid
Whilst FA and its derivatives show excellent affinities to FR in the nanomolar range, solely pteroic acid shows a significant drop in affinity (Kamen and Capdevila 1986; Leamon et al. 2009). Interestingly, it has been shown that pteroic acid conjugated to moieties other than glutamic acid can show affinities in the low nanomolar range again. Figure 2 shows three radiolabeled examples that were developed for SPECT imaging with 111In and 99mTc and which exhibited promising results (Ke et al. 2005; Guo et al. 2011; Müller et al. 2004).
A range of 18F-fluorinated FA derivatives show a tendency for improved in vivo behavior correlated to higher hydrophilicity of the substance (Ross et al. 2008). Therefore, polarity seems to be a critical property for the design of an ideal imaging agent for FR. To combine facilitated synthesis with the benefits of positron emission tomography (PET) as well as the good availability of a generator-based nuclide, we aim to develop 68Ga-labeled pteroic acid-based agents. As the lack of glutamic acid decreases the hydrophilicity of the compound, it is unclear to date whether the introduction of the chelator (DOTA) would be sufficient to compensate the lost polarity. To investigate this systematically, a range of different spacers between the pteroic acid and the chelator are necessary—herein we report synthesis and preliminary in vitro evaluation of the first derivative, which is based on an ethyl spacer and will be used as reference for further compounds.
2 Results and Discussion
2.1 Synthesis
The synthetic route to the desired compound can be seen in Figs. 3 and 4. As FA is known to be sensitive to light and air and shows very poor solubility, we decided to introduce pteroic acid as late as possible into the molecule. The synthesis of an amine-bearing chelator 6 in five steps started with the Cbz protection of 2-aminoethanol and its subsequent bromination under Appel conditions. As the coupling to tris(tert-Bu)DO3A displayed severe problems regarding purification, compound 3 was connected to 1,4,7,10-tetraazacyclododecane (cyclene) according to Duimstra et al. (2005), yielding the monosubstituted derivative 4 exclusively, because no base was added to the reaction mixture. This was followed by introduction of tert-Bu-acetate side-chains to obtain a potent chelator for 68Ga3+. The Cbz protecting group allows orthogonal deprotection by means of hydrogenation to yield tris(tert-Bu)DO3A-EA 6 as a protected chelator that can be coupled to target molecules that carry an acid function. Tris(tert-Bu)DO3A-EA 6 was successfully coupled to protected pteroic acid. Therefore, a novel peptide-coupling reagent, (1-cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino-carbenium hexafluorophosphate (COMU), was used under typical conditions but the extractive standard workup had to be replaced by removal of the solvent and subsequent column chromatography of the residue. For deprotection we anticipated the need of two separate steps: one under acidic conditions to remove the tert-butyl ester groups from the chelator and a second one under basic conditions to remove the formyl- and dimethyl-aminomethylene groups at the pteroic acid. Interestingly, after incubation of compound 7 for 24 h in 1 M NaOH at 45°C, not only the formyl- and dimethylamino-methylene groups as expected, but also the tert-butyl esters, were already cleaved. The labeling precursor 8 could therefore be obtained in seven steps and overall yield of 8.3%.
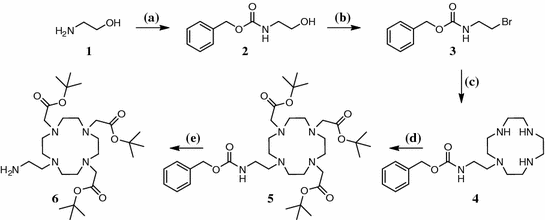

Fig. 3
Five-step synthesis of tris(tert-Bu)DO3A-EA. a H2O, Na2CO3, Cbz-Cl, 0°C, 2 h; b THF, CBr4, PPh3, 0°C—rt, 2.5 h; c toluene, cyclene, reflux, overnight; d MeCN, α-bromo-tert-butyl acetate, Na2CO3, reflux, 24 h; e MeOH/TEA, H2/Pd, rt, 6 h

Fig. 4
Coupling and deprotection. f DMF, COMU, DIPEA, 0°C—rt, 3 h; g 1 M NaOH, 45°C, 24 h
2.2 Radiolabeling and In Vitro Studies
The eluate from a 68Ge/68Ga generator was purified via the cation exchange resin-based process (Zhernosekov et al. 2007) prior to use. A stock solution of the precursor in ethanol was prepared (0.5 mg/ml). Labeling was performed in 400 μl HEPES buffer (0.13 M) under conventional heating as well as under microwave support. Conventional heating was performed at 95°C, and different amounts of precursor (10, 20, 30 nmol, respectively) were investigated. The obtained results are shown in Fig. 5. Best results were obtained with 30 nmol after 10 min, yielding 75% radiochemical yield. Under microwave support the same results could be obtained, but only 10 nmol precursor was necessary and the reaction time could be considerably reduced to only 3 min.
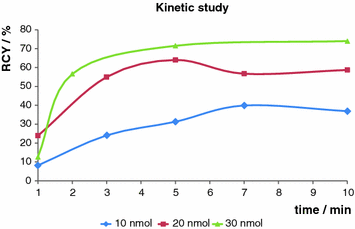

Fig. 5
Kinetic study. Labeling in HEPES (0.13 M) buffer at 95°C
For any in vivo application of a tracer, in vivo stability is essential. Therefore, we examined the complex’s stability in PBS buffer as well as its behavior upon incubation with excessive transferrin. The results obtained were very promising, as in both cases no relevant degradation or transchelation could be detected for up to 3 h (Fig. 6).
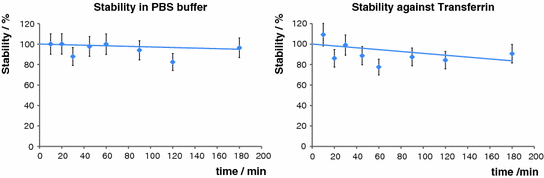

Fig. 6
Stability studies in PBS buffer and against transferrin at 37°C
Finally, the lipophilicity of the new tracer was determined by 1-octanol/PBS extraction. The organic phase was re-extracted twice, and the values of the first extraction were discarded. The obtained logD value of −0.1 ± 0.1 hints that the introduction of DOTA already adds reasonable values of polarity to the molecule, which is necessary for favorable in vivo behavior. It will be proven in in vivo experiments (μPET imaging) whether this is already enough or still needs to be improved.
3 Conclusion
To develop a pteroic acid-based PET tracer, synthesis of 2,2′,2′′(10-(2-(4-((2-amino-4-oxo-3,4-dihydropteridin-6-yl)methylamino)benzamido)ethyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetic acid 8 could successfully be achieved in seven steps. The conditions for labeling with 68Ga have been investigated—good radiochemical yields can be achieved in 0.13 M HEPES buffer at 95°C for 10 min using 30 nmol of compound 8 or under microwave support with only 10 nmol precursor in only 3 min at 300 W. The 68Ga complex is stable in PBS buffer as well as in the presence of excessive transferrin for at least 3 h. The logD value of −0.1 ± 0.1 for our novel tracer is in a range that allows favorable in vivo characteristics for PET imaging of FR to be assumed. After determining the affinity of our new 68Ga radiotracer(s) to FR using human KB cells, they will be subject to further studies using in vivo μPET imaging.
4 Experimental
4.1 Benzyl-N-(2-hydroxyethyl)carbamate
To a cooled solution of 1.5 g (24.5 mmol) 2-aminoethanol in 15 ml water, 12.5 ml Na2CO3 (2 N) was added alternately with 3.5 ml benzylchloroformate (24.5 mmol) within 40 min. The mixture was allowed to stir for further 80 min at 0°C and was then extracted with ethyl acetate. After washing the organic phase with water, saturated NaHCO3 solution, and brine, it was dried over Na2SO4. The solvent was evaporated to give the product as 3.45 g (17.7 mmol, 72%) of white crystals. 1H-NMR (300 MHz, CDCl3), δ [ppm] = 2.39 (1H, s, br, –OH), 3.32 (2H, m –NCH 2), 3.68 (2H, t, J = 5.06 Hz, –CH 2OH), 5.08 (2H, s, –CH 2–C6H5), 5.22 (1H, s, br, NH), 7.35–7.28 (5H, m, –CH2–C6 H 5).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree