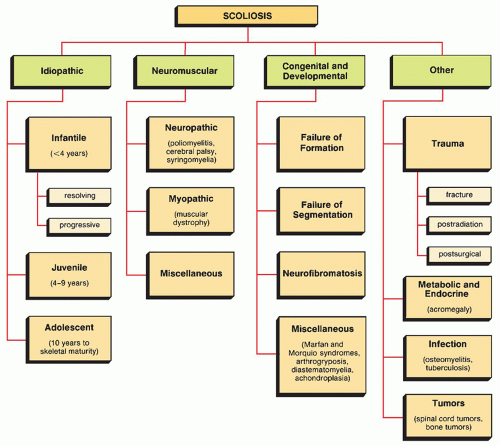
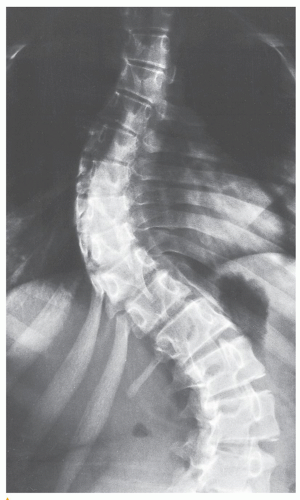
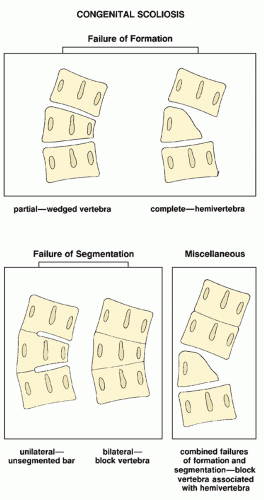
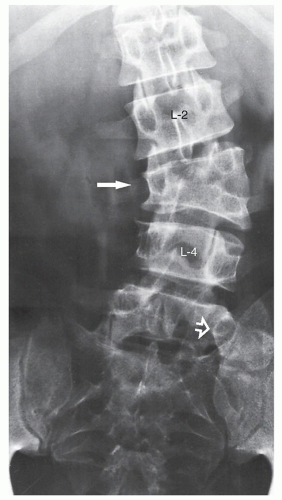
which has been adopted and standardized by the Scoliosis Research Society, classifies the severity of scoliotic curvature into seven groups (Table 33.2).
TABLE 33.1 Standard Radiographic Projections and Radiologic Techniques for Evaluating Scoliosis | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||
TABLE 33.2 Lippman-Cobb Classification of Scoliotic Curvature | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
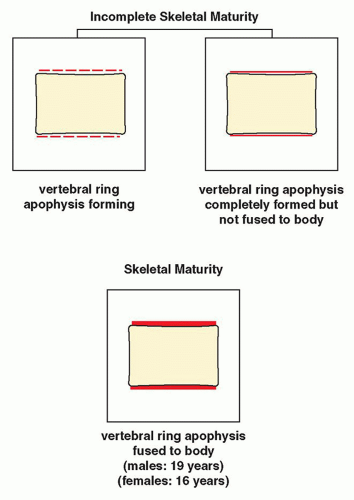 FIGURE 33.13 Skeletal maturity. Determination of skeletal maturity from ossification of the vertebral ring apophysis. |
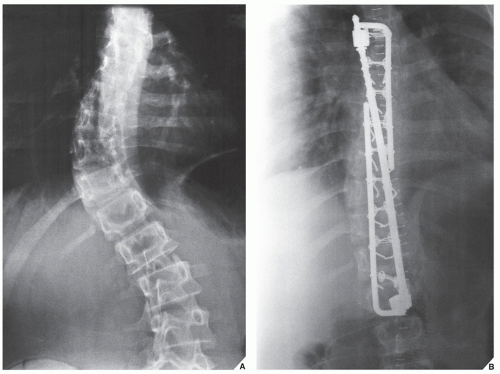
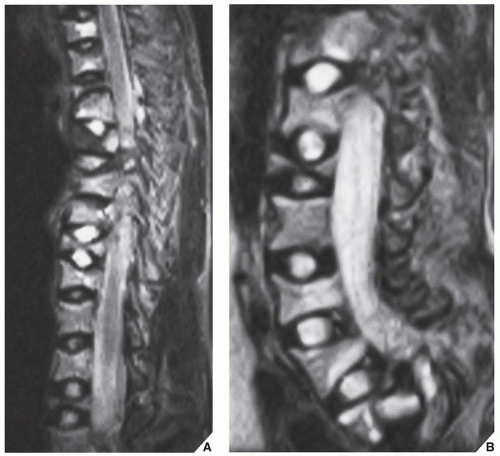
base of the spinous process at each level of the spine to be fused, are then fixed to the L-rods. Variations in this technique have been used with L-rod instrumentation alone, which involves the use of sublaminar wires fixed to the rods, or a combination of Harrington distractors and wires fixed to them. Cotrel-Dubousset spinal instrumentation using knurled rods has also gained popularity. Fixation is achieved via pediculotransverse doublehook purchase at several levels. The two knurled rods are additionally stabilized by two transverse traction devices. The Dwyer technique, involving anterior fixation of the spine and obliteration of the intervertebral disks, is also used in the surgical treatment of scoliosis but more often in the paralytic types of the deformity.
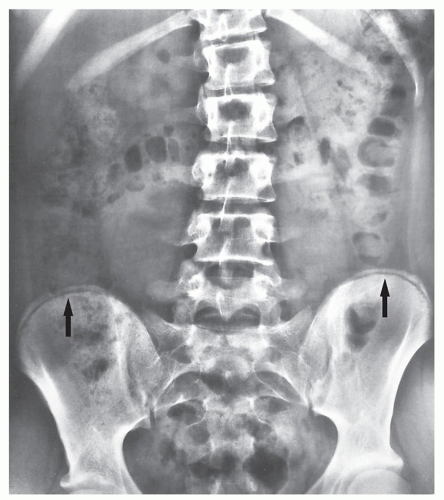
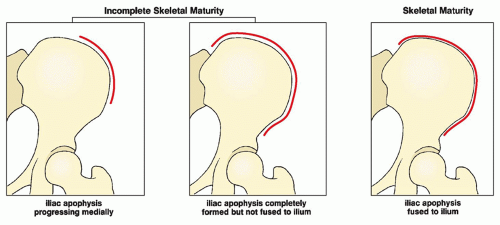 FIGURE 33.15 Skeletal maturity. Determination of skeletal maturity from the status of ossification of the iliac apophysis. |
and café-au-lait spots, that may be present at birth or may appear over time, occur in more than 90% of patients. The latter lesions have a smooth border that has been likened to the coast of California; this distinguishes them from the café-au-lait spots seen in fibrous dysplasia, which have rugged “coast of Maine” borders. These spots increase in size and number as the person grows older. Axillary or inguinal freckles are rare at birth but appear throughout childhood and adolescence. Plexiform neurofibromatosis is a diffuse involvement of the nerves, associated with elephantoid masses of soft tissue (elephantiasis neuromatosa), and localized or generalized enlargement of a part or all of a limb (Fig. 33.17). Patients with these manifestations are particularly prone to malignant tumors (see Fig. 22.40).
TABLE 33.3 Most Effective Radiographic Projections and Radiologic Techniques for Evaluating Common Anomalies with General Affliction of the Skeleton | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
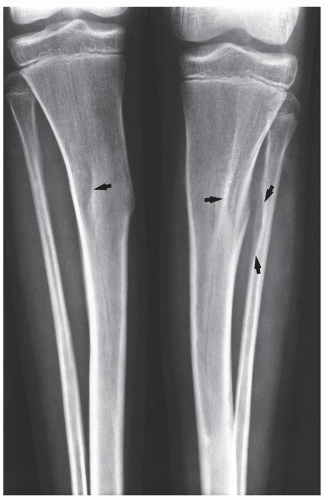
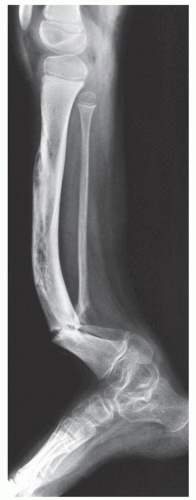
child syndrome (“shaken baby syndrome,” parent-infant trauma syndrome) are (a) the presence of blue sclera or abnormal teeth in OI, (b) investigation of clinical and family history (invariably positive in OI), (c) physical examination, and (d) radiologic examination for the detection of wormian bones and osteoporosis in OI; and metaphyseal corner fractures and “buckethandle” fractures that are highly specific and virtually pathognomonic features of child abuse. Several other features are also specific for child abuse, including multiple rib fractures, especially posterior rib fractures near or at the costovertebral junction; multiple fractures and/or multiple fractures showing different stages of healing; and sternal or scapular fractures, especially of the acromion. Transverse, oblique, or spiral fractures of a long bone with normal mineralization in the absence of any previous history, especially in a nonambulatory infant, are also highly suggestive of child abuse. The key to diagnosis of either condition is the correlation of clinical history, physical examination, family history, and imaging findings.
 FIGURE 33.25 CT of osteogenesis imperfecta. Axial CT sections of the skull (A) through the frontal and parietal bones and (B) through the vertex show sutural (wormian) bones. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree