chapter 9 Small Cell Lung Cancer
INTRODUCTION
Small cell lung cancer (SCLC) comprises about 20% of lung cancers diagnosed in the United States. Its biology is characterized by rapid proliferation rate and, unlike non-small-cell lung cancer (NSCLC), has exquisite sensitivity to chemotherapy. While the TNM staging system can be applied to SCLC, most patients are served well by the dichotomous Veterans Administration Small Cell Lung Cancer Staging system, which divides patients into a limited or extensive stage. Patients whose known disease is limited to one hemithorax (or as in another definition, those whose disease could be treated with “reasonable” radiation fields) are considered to have limited-stage disease. However, as experience has shown, most of these patients already have occult distant metastases and local therapy alone is unlikely to cure them. Before the availability of chemotherapy, patients were treated with radiotherapy or surgery with almost invariably poor outcome. When chemotherapy became available, impressive responses were seen, but lasting remissions were rare due to the emergence of resistant clones. The role of radiotherapy in limited-stage SCLC was debated until two meta-analysis reports showed a small but significant radiotherapy contribution to patient survival.1,2
The modern paradigm for treatment of limited-stage SCLC includes the combination of radiotherapy and chemotherapy. There appears to be some advantage to giving radiotherapy early in the course of chemotherapy if administered with cisplatin and etoposide.3,4 The radiobiology of SCLC suggests that hyperfractionated and accelerated radiotherapy may be beneficial, and indeed, a prospective randomized study showed improved survival in patients who received 45 Gy over 3 weeks, compared to those who received the same dose over 5 weeks.5 However, this hyperfractionated regimen is associated with a high rate of acute esophageal toxicity and there is a possibility that a higher radiation dose given in once-daily treatments may result in equal outcome, with less toxicity.
FDG-PET in Staging of Small Cell Lung Cancer
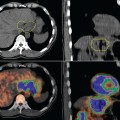
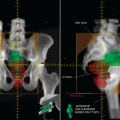
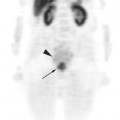
Until recently, clinical information on FDG-PET imaging in SCLC has been scarce. Although lung cancer was one of the earliest indications for PET imaging, the vast majority of data has been obtained on NSCLC. As of the time of this writing, the use of FDG-PET for SCLC is still considered experimental by the Center for Medicare and Medicaid Services and not reimbursed outside of a clinical trial. However, the aggressive biology of SCLC suggests that it should be just as FDG-avid as NSCLC. Emerging clinical data on SCLC thus far support this hypothesis. Several smaller studies initially suggested that FDG-PET is, at the very least, comparable to the standard staging modalities for SCLC.6–10 Two prospective studies have recently been published. Brink and colleagues studied 120 newly diagnosed patients with SCLC, comparing findings obtained by whole-body PET to the results obtained using standard staging procedures (history and physical, CT, bronchoscopy, bone scan, bone marrow biopsy, and cranial CT or magnetic resonance imaging [MRI]).11 Discrepant findings were further investiga-ted by other imaging modalities or biopsied whenever possible. If the results of additional examinations were still equivocal, consensual diagnosis was made by a committee of clinicians using data that included follow-up observations. The sensitivity of FDG-PET for primary tumor, extrathoracic lymph nodes, and non-brain distant metastases was 100%, 100%, and 98%, respective-ly, compared to 100%, 70%, and 83% for the conventional workup. The sensitivity of PET for brain metastases was rather low at 46%. Specificity of FDG-PET for lymph nodes and distant metastases was 98% and 92% compared to 94% and 79% for conventional staging studies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree