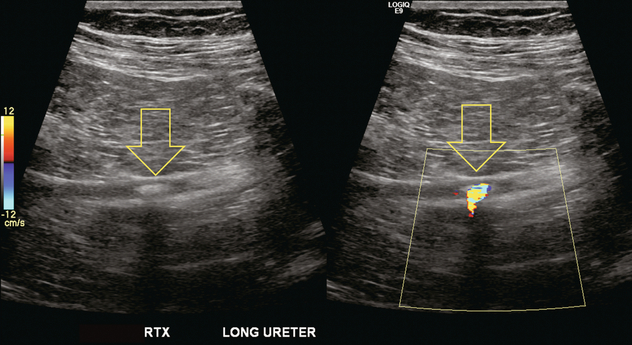
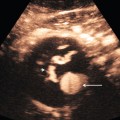
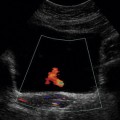
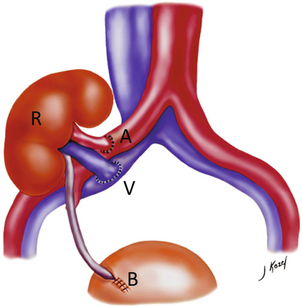
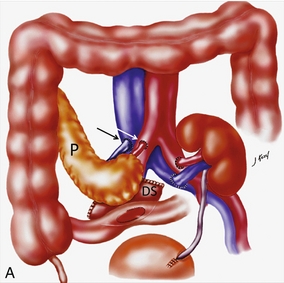
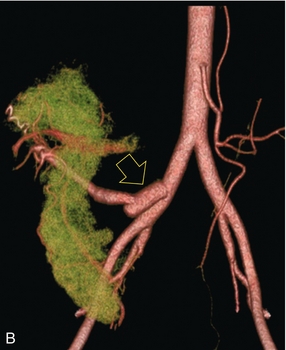
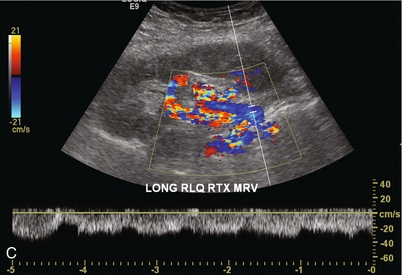
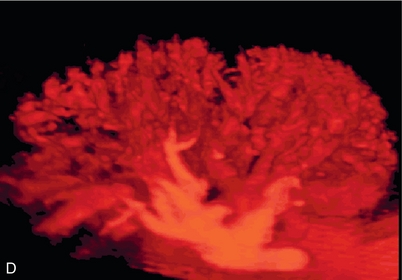
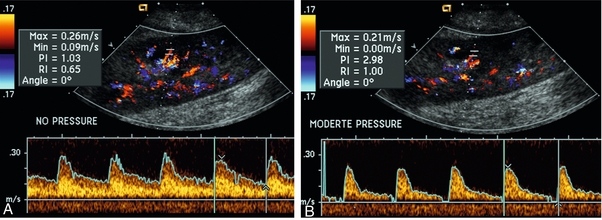
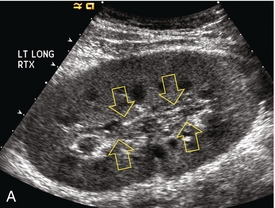
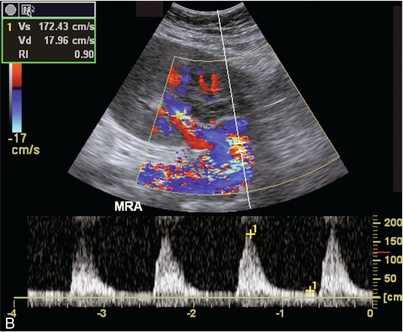
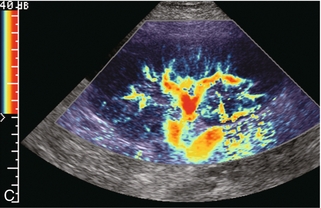
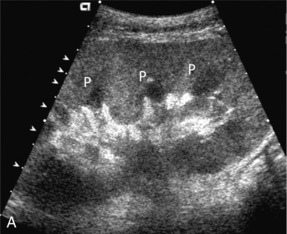
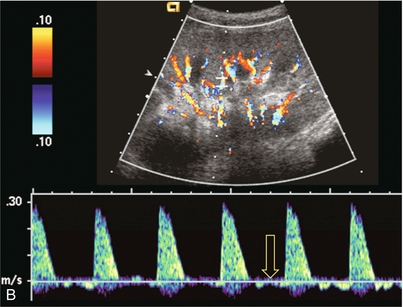
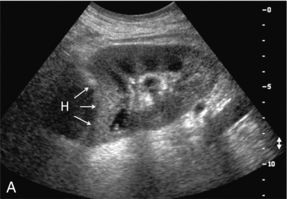
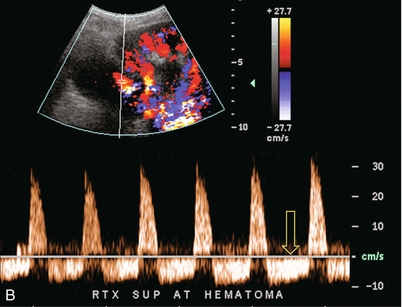
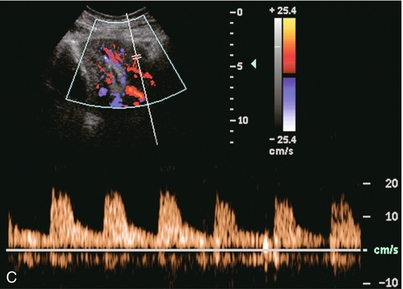
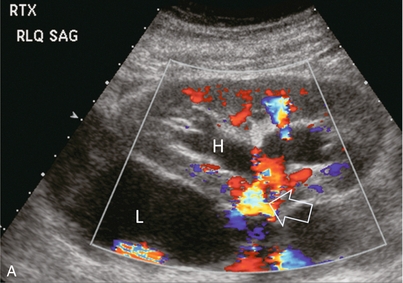
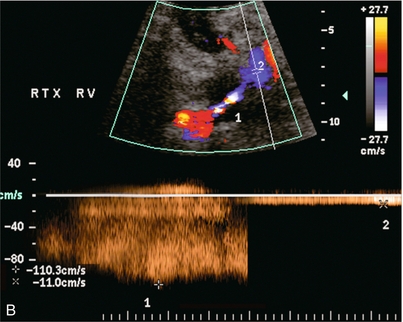
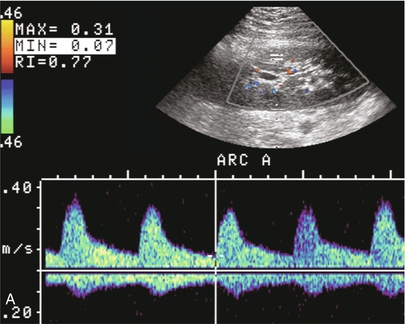
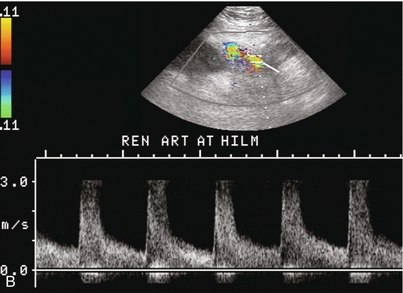
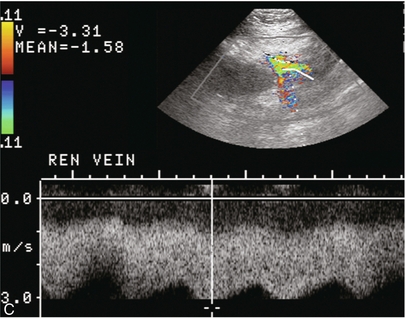
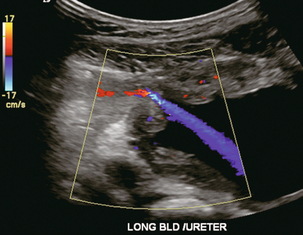
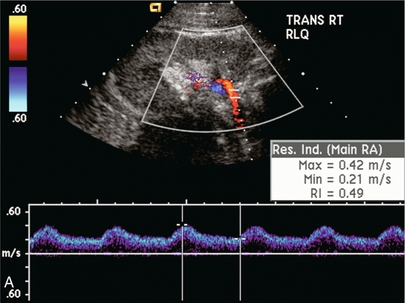
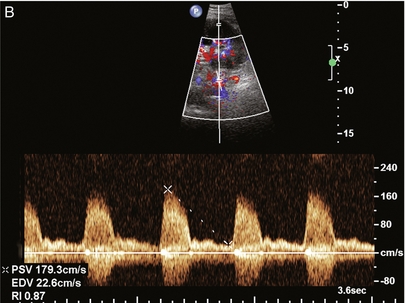
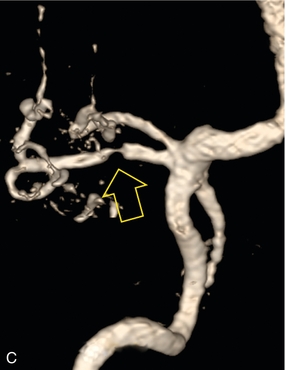
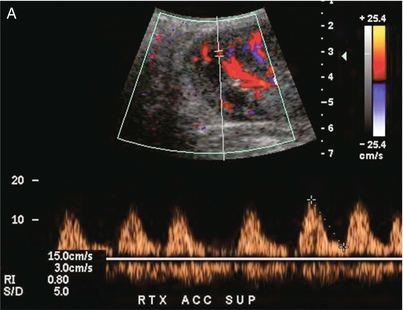
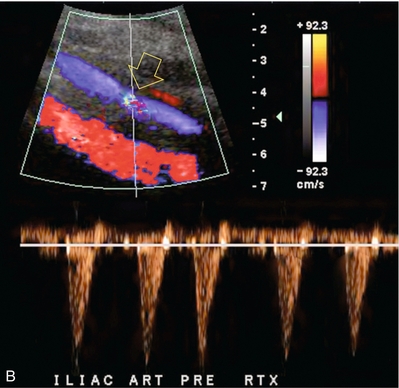
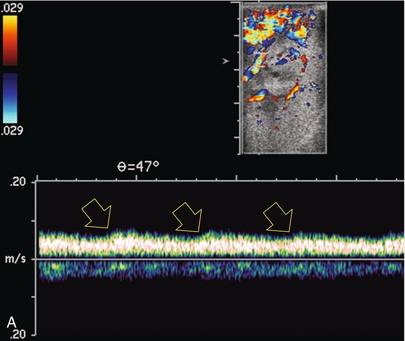
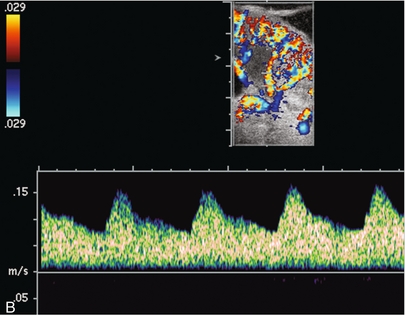
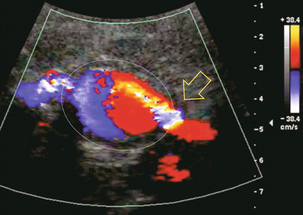
Chapter 10 The number of transplant candidates on waiting lists for organs continues to increase each year. The shortage of organs remains a major problem for patients with end-stage liver, renal failure and diabetes mellitus. Graft and patient survival rates following solid organ transplantation continue to improve due to refinements in surgical technique, advances in human leukocyte antigen typing for recipient/donor matching1 new and improved immunosuppressive agents2,3 refinements in national coordinated organ-sharing systems, improvements in recipient immune system desensitisation and advances in non-invasive transplant monitoring. As the number of transplant recipients grows, it is important for anyone responsible for providing imaging services to at least have a basic understanding of transplantation and its complications since there is an increasing incidence of transplant recipients presenting for emergency care to non-transplant centres. Chances for graft survival are significantly improved with timely identification of the aetiology of transplant dysfunction, allowing prompt medical and/or surgical intervention when necessary. Quality of life after successful renal transplantation significantly improves for patients in dialysis-dependent end-stage renal disease. Renal allograft and patient survival rates continue to improve. In the United States 1-year graft survival currently approximates 91.2% for deceased donor and 96.3% for living-donor kidney transplants.4 When screening laboratory test results indicate renal transplant dysfunction, imaging studies are often required to evaluate renal morphology and perfusion. Doppler ultrasound is an ideal tool for this purpose because it is non-invasive, readily available, and can detect and distinguish many of the vascular complications that can be the cause of transplant dysfunction. The application of Doppler ultrasound to functional problems, such as rejection or acute tubular necrosis, however, is limited. Examination of the transplant kidney requires careful attention to scan technique and awareness of potential pitfalls. A complete sonographic examination of the renal transplant should cover the points listed in Box 10-1. The most common abnormal findings, which may be demonstrated, are listed in Box 10-2. In most cases, the isolated transplant kidney is positioned retroperitoneally in the right iliac fossa with an end-to-side anastomosis of the renal vasculature to the common or external iliac artery and vein (Fig. 10-1). If the patient has undergone a simultaneous renal–pancreas transplant, then the kidney is usually intraperitoneal within the left flank. The transplanted ureter is implanted directly into the superior surface of the bladder or to the native ureter (see Fig. 10-34A). In approximately 20% of transplants, because of variation in donor anatomy, multiple arterial or venous anastomoses may be required. Because numerous technical variations exist in the way kidneys are transplanted, it is very important that the sonologist is familiar with the surgical technique common to their institution and the specific anatomical details of the patient being scanned. If the patient’s surgical anatomy varies from standard, proper documentation and communication of the surgical record is very important in ensuring correct understanding and interpretation of imaging findings and Doppler flow profiles. Ideally, if the transplant has variant vascular anatomy, a drawing is recorded by the surgical team which shows the orientation of the kidney and its vasculature, the number and location of anastomoses, and any other atypical anatomical information. FIGURE 10-1 Artist rendering of a renal transplant (R) located in the right iliac fossa. The transplant renal artery is typically anastomosed to the common iliac artery (A). The transplant renal vein anastomosis is to the common iliac vein (V). The ureter is connected to the urinary bladder (B). FIGURE 10-34 Artist rendering of a combined renal pancreas transplant. (A) Typically the pancreas (P) is transplanted on the right with an interposed duodenal stump (DS) between pancreas and ileum. The splenic artery and vein, which serve as the vascular pedicle for the pancreas transplant, are anastomosed to the common iliac artery and inferior vena cava. (B) With multiorgan donation harvesting of the vascular pedicle with the liver often leaves the pancreas requiring an interposition graft. This is typically accomplished with donor iliac artery. Branches are connected to the remnants of the splenic and gastroduodenal arteries, perfusing the body and the head of the pancreas respectively. Interposition jump grafts can be quite creative with the goal of establishing good perfusion to the end organ. Again, transmission of the surgical record is critical to the imager trying to understand complex surgical anatomy to the organ they are being asked to study. 3-D CTA of a pancreas transplant with an interposition graft added to establish appropriate length of the arterial conduit. Ultrasound examination of the kidney should first confirm its appropriate location and the absence of any significant fluid collections. Colour Doppler is then used to identify the renal vascular pedicle. Spectral Doppler is applied to the main renal artery, main renal vein and intrarenal segmental or intralobar branches at the mid, upper, and lower poles. If any inflow compromise is suspect to all or part of the kidney, then power Doppler can be applied to confirm uniform vascular perfusion to the organ5 (Fig. 10.2A–D). FIGURE 10-2 Longitudinal colour, spectral and power Doppler images of a normal renal transplant. (A) Colour Doppler image of the vascular pedicle reveals the anastomosis. The vessels often become curved in their course as the organ is nestled into the iliac fossa. (B) spectral Doppler tracing of the renal artery shows a brisk upstroke in systole. The resistive index is appropriate at between 60 to 70%. (C) Normally, the renal vein flow profile shows an element of cardiac periodicity. This presence of periodicity in the renal vein helps reinforce anastomotic patency. (D) 3-D rendering of a power Doppler evaluation shows uniform distribution of flow out to the capsular surface of this normal renal transplant. The gain should be set at the highest level possible without creating noise in the image or tracing. To ensure proper perception of flow by colour Doppler, or accurate measurement of the spectral velocity, the angle of insonation should be less than 60ᵒ. Finding an appropriate angle can be especially problematic when examining transplanted kidneys because their vessels may be extremely tortuous and a committed search for a suitable Doppler window is required.6 Often the imaging study is limited because intervening adipose tissue increases the distance from the patient’s skin to the transplanted kidney or there is gas in overlying bowel. By applying sufficient pressure, fat or bowel loops can be displaced. Doing so, however, will compromise the Doppler examination as the renal parenchyma also becomes compressed and inflow can be impeded (Fig. 10-3). This results in elevation of the resistive index. Thus, care must be taken not to apply excess pressure to the kidney or its associated vasculature, so that any diagnosis made on the basis of the resistive index or velocity measurement is accurate.7 FIGURE 10-3 (A) Renal transplant interlobar artery spectral Doppler tracing acquired with gentle transducer contact. Note the normal waveform and 65% resistive index. (B) When moderate pressure is applied with the transducer, the tracing of the same artery now exhibits an elevated resistive index of 100%. Transducer pressure alone is responsible for this increase in vascular impedance and resultant elevation of resistance. Functional complications include hyperacute rejection, perioperative ischaemia, acute tubular necrosis, acute rejection, chronic rejection and drug toxicity (most commonly immunosuppressive agents). Imaging techniques, including ultrasound with Doppler, are limited in their ability to identify and distinguish these complications.8 Acute rejection is a cellular-mediated process, whereby the immune system attacks the foreign renal allograft. Acute rejection is controlled by the use of steroids, cyclosporine, tacrolimus, sirolimus and other immunosuppressive agents. Occasional elevation in a transplant recipient’s immune status (triggered by viral illness or non-compliance with the immunosuppressive drug regimen) can result in an acceleration of acute rejection to a critical level. The kidney becomes oedematous and swollen, intracapsular pressure rises, and eventually resistance to vascular perfusion increases (Fig. 10-4). Although early investigators proposed that resistive index elevation was useful in identifying acute rejection as the cause of kidney transplant dysfunction, subsequent laboratory and clinical studies have shown it to be unreliable, and acute rejection remains a pathological diagnosis. Indeed, in a canine study it has been found that resistive index actually decreases in the mild to moderate stages of acute rejection.9 During the early to mid-stages of rejection, the physical effects of increased intrarenal pressure are counteracted by intrarenal hormonal autoregulatory mechanisms. Elevation of resistive index, therefore, does not manifest until the process of acute rejection is quite severe.9 If a scan being performed in anticipation of transplant biopsy identifies the kidney to be oedematous and swollen with loss of central sinus fat echo and very high resistive indices, thought should be given to deferring the biopsy. Puncturing the capsule of a tense rejecting kidney may cause it to rupture. FIGURE 10-4 (A) Longitudinal grey scale image of a transplanted kidney. Note the rounded globular configuration of the kidney. The central hilar space (arrows) is compressed due to oedema and swelling; and the central hilar fat has been displaced. (B) Spectral Doppler tracing at the main renal artery level shows a resistive index of 90%. Biopsy confirmed severe acute rejection. (C) Power Doppler image of a severely rejecting renal transplant shows the main central vasculature and a few interlobar vessels but no flow can be seen out to the periphery. The high resistance generated by the rejection process results in this colour Doppler ‘pruned tree’ appearance. Contrast this to the appearance of normal renal power Doppler flow in Figure 10-2D. Resistive index is rarely affected in the mild to moderate stages of acute rejection and when it is, its specificity is low. It is not until acute rejection progresses to severe levels that the resistive index becomes consistently elevated. Elevation of resistive index, however, can also occur from many other causes such as hydronephrosis, acute tubular necrosis, infection and compression of the kidney by an adjacent mass or fluid collection. Thus, specificity for the diagnosis of acute rejection by Doppler ultrasound is unacceptably low and renal biopsy is still needed to establish the diagnosis.9,10 Chronic rejection is a multifactorial process, mostly antibody-mediated, but the pathophysiology is not entirely understood. Doppler indices rarely show any significant alteration in flow profiles with chronic rejection.11 Perioperative ischaemia can result in transient compromise of renal function, particularly at the level of the distal tubules which are most sensitive to hypoxia. This condition is self-limiting and typically resolves within 1–2 weeks of transplantation. The medullary pyramids are oedematous and therefore appear enlarged and hypoechoic, and spectral Doppler shows an increase in the resistive index. Although the imaging and Doppler findings may suggest acute tubular necrosis, specificity is low9 (Fig. 10-5). FIGURE 10-5 (A) Longitudinal grey scale image obtained with 24 hours of implantation. This deceased donor organ experienced prolonged ischaemic time. Note that the kidney has an appropriate contour and the sinus fat is preserved. The medullary pyramids (P), however, are prominent, hypoechoic and oedematous. (B) Spectral and colour Doppler image of the same kidney. The resistive index approximates 100%. This combination of findings in the appropriate clinical situation is consistent with acute tubular necrosis. This is common immediately after transplantation but can also be seen with drug toxicity. These include haematomas, seromas, urinomas, abscesses, lymphoceles, obstructive hydronephrosis, focal masses, arterial and venous stenosis or thrombosis, and intrarenal arteriovenous fistula and pseudoaneurysm.12,13 Unlike functional complications, most anatomic complications are readily identified by ultrasound. Perinephric fluid is a common sequela of renal transplantation and is not considered significant if it is crescentic in shape, and decreases in size over time. Most fluid collections are haematomas or seromas, which weep from tissue around the transplant. Urinomas are relatively uncommon and usually are the result of breakdown at the ureteral anastomosis to the bladder. Doppler examination is of limited value in these cases. High-pressure collections, such as haematoma after biopsy or organ rupture may exert mass effect upon the kidney and locally affect haemodynamics. In this case, Doppler may reveal a high-resistance spectral tracing adjacent to the fluid pocket (Fig. 10-6). FIGURE 10-6 (A) Longitudinal grey scale image of a renal transplant within 24 hours of an upper pole biopsy. The biopsy was complicated by haemorrhage (H). Blood accumulated in the subcapsular space and severely compressed the upper pole of this kidney (arrows). (B) Spectral Doppler tracing obtained of an interlobar artery just adjacent to the high-pressure haematoma reveals extremely high resistive index with reverse flow in diastole. (C) Spectral Doppler tracing at the opposite (lower pole) of this same kidney shows a normal low resistance flow profile. The compressive haematoma exerts local mass effect and elevates resistance to flow. Lymphoceles usually manifest at about 6–8 weeks postoperatively and are seen as rounded, lobulated collections near the vascular anastomoses. They are the result of surgical disruption of iliac lymphatic channels when the vascular anastomosis to the transplanted kidney is created. An expanding lymphocele may cause ureteric compression and hydronephrosis. If a lymphocele becomes large enough, it may compress or kink the renal vascular pedicle. In this situation, Doppler examination may show findings similar to arterial or venous stenosis14 (Fig. 10-7). FIGURE 10-7 (A) Longitudinal colour Doppler image of this renal transplant shows a large fluid collection medial to the kidney, surrounding the renal vascular pedicle. This is a lymphocele (L) and it has caused distortion of the pedicle. Colour aliasing can be seen in the renal artery (arrow). There is also obvious hydronephrosis (H) caused by compression of the ureter. (B) Spectral Doppler tracing of the renal vein shows marked compression where it courses past the lymphocele (#1), the measured velocity at this area is 1.1 m/s; whereas within the kidney (proximal to the lymphocele (#2)) the velocity is only 0.1 m/s. This 10-fold velocity gradient identifies this as a significant renal venous outflow obstruction. Intraperitoneal renal transplantation typically when combined with pancreas transplantation results in a mobile kidney. Occasionally ptosis or rotation and torsion of the kidney may occur along its vascular pedicle. This may result in arterial inflow and venous outflow narrowing or obstruction15 (Fig. 10-8). FIGURE 10-8 (A) Colour Doppler image along the long axis of an intraperitoneal renal transplant with a spectral Doppler tracing of an arcuate artery. The waveform shows a tardus parvus configuration indicating arterial inflow compromise. (B) Doppler of the main renal artery at the hilum reveals a high-velocity jet approximating 3 m/s. (C) Immediately adjacent to the main renal artery, the main renal vein tracing also shows turbulent high-velocity flow. This finding of high-velocity, turbulent Doppler tracings in both artery and vein identifies partial torsion of the renal vascular pedicle with a twisting compromise of the main artery and vein. Fortuitously this was only partial torsion of the kidney and was corrected with surgical detorsion and nephropexy. Torsion of an intraperitoneal transplant kidney has been reported to result in infarction. Transient dilatation of the collecting system as a result of ureteral anastomotic oedema frequently occurs immediately after renal transplantation or removal of the ureteral stent. The presence of a dilated transplant collecting system does not automatically signify an obstructed system under pressure, as the denervated, flaccid collecting system can become markedly dilated, particularly when the urinary bladder is distended. Platt et al.16 proposed that the identification of an elevated resistive index was useful in distinguishing obstructive hydronephrosis from chronic, low-pressure dilatation of the transplant collecting system. Although this observation may be sensitive, its specificity is very poor because of the many other factors that similarly affect renal haemodynamics. Nuclear scintigraphy or the Whitaker test are more specific for differentiating high-pressure obstructive hydronephrosis from low-pressure distention of a flaccid renal transplant collecting system. The transplanted ureter is normally anastomosed to the superior surface of the bladder. Occasionally, colour Doppler can identify the ureteral jet from the transplanted ureter. The posteriorly directed flow of urine may initially confuse the unexperienced examiner (Fig. 10-9). FIGURE 10-9 Colour Doppler image of the urinary bladder. The transplanted ureter is normally anastomosed to the anterior superior bladder wall. Flow of urine is seen through the anastomosis manifesting as a jet, but pointing posteriorly; not in the direction we are accustomed to seeing ureteral jets. Although uncommon, urinary tract calcifications can develop within a transplant kidney. When evaluating a renal transplant with hydronephrosis, the dilated ureter should be followed toward the anastomosis. If intraluminal debris is identified. The application of colour Doppler may reveal twinkle artifact. This helps identify the abnormality as a calculus (Fig. 10-10). Following renal transplantation, vascular complications are observed in less than 10% of recipients; however, when present, they are associated with a high morbidity and mortality. Complications include renal artery or vein stenosis, compression, kink, thrombosis, intrarenal arteriovenous fistula and pseudoaneurysm. If identified promptly, they can often be successfully repaired prior to transplant failure. Doppler sonography is a very effective, non-invasive tool for identifying significant vascular complications.17–19 This is most often observed within 1–2 cm of the anastomosis, usually as a result of vessel wall ischaemia due to disruption of the vasa vasorum within the artery wall. Stenosis should be suspected if a tardus parvus waveform and relatively low-resistance flow are noted in the intrarenal branches. A tardus parvus waveform is characterised by a delayed upstroke in systole (prolonged acceleration time > 0.07 s), rounding of the systolic peak and obliteration of the early systolic notch. A flow velocity approximating 2 m/s with associated distal turbulence near the renal artery anastomosis is diagnostic of renal artery stenosis (Fig. 10-11). If an intrarenal tardus parvus waveform is observed, but a stenosis cannot be identified at the level of the renal artery anastomosis, the examiner should conduct a thorough examination from the renal hilum through the iliac artery in search of inflow compromise (Fig. 10-12). With severe renal artery stenosis the intra-arterial waveforms become flattened to the point that systolic diastolic velocity variation becomes difficult to perceive. Subtle pulsatile flow is enough to document the patency of the artery and avoid misdiagnosis of thrombosis. This is further reinforced by identifying outflow in the renal vein (Fig. 10-13). FIGURE 10-11 (A) Renal transplant Doppler tracing in a hypertensive recipient with elevated serum creatinine. The renal arterial waveform manifests tardus parvus configuration with a slow systolic upstroke, and a rounded systolic peak with low resistance. These findings suggest a more proximal stenosis. (B) Spectral Doppler tracing closer to the anastomosis of the main renal artery to the iliac artery. Note the high-velocity (1.79 m/s), turbulent flow characteristic of renal artery stenosis. (C) MR angiogram of this transplanted kidney actually revealed a double arterial anastomosis. The lower pole artery has a high-grade stenosis (arrow) within 1 cm of its anastomosis. FIGURE 10-12 (A) Spectral Doppler tracing of a transplant main renal artery. Tardus parvus changes were identified but an anastomotic stenosis could not be identified. (B) The exam was extended to include a spectral Doppler tracing of the external iliac artery. A high-velocity jet is identified with velocities of > 3 m/s. An atheromatous lesion in the right iliac artery in this diabetic recipient was responsible for the inflow compromise to the renal transplant. FIGURE 10-13 Intraoperative spectral and colour Doppler ultrasound of a recent renal transplant recipient being reoperated because of poor renal function. Preoperative Doppler examination suggested arterial stenosis. (A) Direct contact scanning on the kidney showed an abnormal spectral Doppler tracing of a segmental artery with only a very subtle undulation in the arterial waveform (towards the transducer). A high-grade proximal inflow compromise wiped out the expected systolic/diastolic arterial velocity variation. (The tracing below the baseline is an adjacent vein.) (B) A kink of the vascular pedicle was identified and as the artery was surgically manipulated the spectral Doppler tracing suddenly normalised. A long-standing undiagnosed stenosis can develop post-stenotic dilatation. Imaging and colour Doppler reveal a characteristic focal area of expansion of the artery. This usually incorporates the high-velocity post-stenotic jet (Fig. 10-14). FIGURE 10-14 Colour Doppler image along the long axis of the main renal artery. A focal area of stenosis with a high-velocity jet presents as focal turbulence and aliasing. Sacular dilatation of the transplant artery represents a post-stenotic aneurysm and reveals a swirling flow pattern. Flow continues on towards the kidney in the more distal normal-calibre artery. Approximately 20% of transplanted kidneys require more than one arterial anastomosis due to the presence of accessory arteries. If one of these vessels becomes compromised, then perfusion to the subtended segment of the kidney is decreased. Again, a tardus parvus waveform is seen, this time limited to the segment perfused by the affected artery. If thrombosis of this artery occurs, then the subtended area shows no flow on colour or power Doppler and an arterial tracing will not be identified by spectral Doppler. The area affected will vary depending on the anatomic vascular distribution (Fig. 10-15). FIGURE 10-15 Longitudinal power Doppler image of the lower pole of a renal transplant recipient with hypertension. There is complete absence of flow at the lower pole. This renal transplant required two arterial anastomoses. Thrombosis of the lower pole artery was suggested and confirmed at angiography.
Solid Organ Transplantation
Introduction
Renal Transplantation
ULTRASOUND ANATOMY OF THE RENAL TRANSPLANT
DOPPLER ULTRASOUND TECHNIQUE
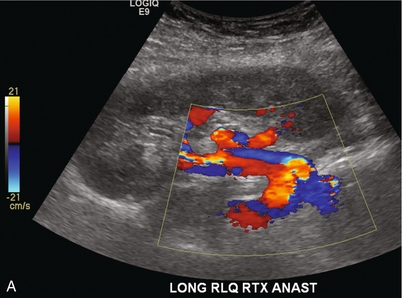
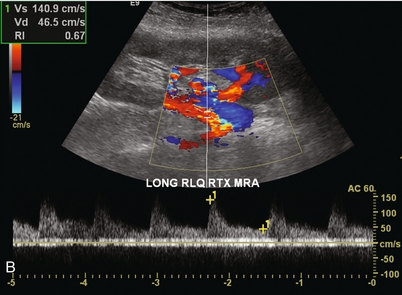
Doppler Gain
Optimising Angle of Insonation Relative to Vessel Orientation
Minimising Transducer Pressure
COMPLICATIONS OF RENAL TRANSPLANTATION
Functional Complications
Anatomic Complications
Vascular Complications
Renal Transplant Artery Stenosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree