Special Issues Regarding Interventions in the Spleen
Interventional procedures in the spleen are relatively rare. This is due to a lack of “hard indications” for splenic biopsy but also to the (culturally instilled) attitude of many operators that it is too dangerous to puncture the spleen because of the risk of hemorrhage. This reluctance stems in part from a lack of awareness of necessary indications (benefits) and the risks relating to the proximity of the pleura (risk of a two-cavity procedure) and bowel (risk of injury).1 The technique of percutaneous splenic biopsy and drainage is basically the same as that described for liver biopsies and abscess drainage and need not be detailed here.
23.1 Diffuse Splenic Changes
The differential diagnosis of diffuse splenic changes includes portal hypertension, acute and chronic infectious diseases, tumors, and hemato-oncologic conditions. Potential systemic or malignant causes are diffusely infiltrating lymphomas and myeloproliferative syndromes. Right heart failure, portal vein thrombosis, splenic vein thrombosis, metabolic and storage diseases, and amyloidosis should also be considered. Splenomegaly may have numerous other causes:
Portal hypertension
Myeloproliferative syndrome (chronic myeloid leukemia, polycythemia vera)
Osteomyelosclerosis
Hairy cell leukemia
Storage diseases especially Gaucher disease)
Chronic infections such as kala-azar (leishmaniasis)
Form of anemia with severe hemolysis
Infections, acute
Infections, chronic
Congestive heart failure
Anemia due to hemolysis
Hemato-oncologic disease (except for myeloproliferative syndromes)
Acute and chronic lymphocytic leukemia
Acute myeloid leukemia
Hodgkin and non-Hodgkin lymphoma
Portal vein thrombosis, splenic vein thrombosis
Autoimmune diseases
Storage diseases (except for Gaucher disease)
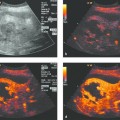
The spleen usually has a homogeneous structure when imaged by ultrasound. Tuberculosis, candidiasis, or syphilis may produce scattered foci or decreased or greatly increased echogenicity.
Ultrasound contrast agents have proven helpful for the exclusion of tiny, circumscribed splenic changes that may indicate malignant or inflammatory infiltration with microabscess formation in the setting of Candida sepsis or other bacterial infections.1–7 It should be emphasized that plain B-mode imaging with a high-resolution transducer (7–17 MHz) can yield equally good results.8 Similar voids may be found in hemophagocytic syndrome (personal data, previously unpublished).
Diffuse splenic changes in themselves are not an indication for splenic biopsy, which would be absolutely contraindicated in patients with portal hypertension, for example.
Possible complications of splenomegaly:
Splenic infarction (splenic abscess)
Splenic capsule rupture with hemorrhage
Splenic fibrosis
Pulmonary ventilation disorders
Note
Diffuse splenic changes are not an indication for splenic biopsy.
23.2 Specific Disorders
23.2.1 Splenic Rupture
Splenic rupture is a threatening condition because it may lead to hemorrhagic shock culminating in death. Splenic injuries usually appear as hypoechoic foci that may be hyperechoic in the acute stage. Splenic rupture may have a traumatic etiology or may occur spontaneously in patients with underlying splenic pathology. Splenic injuries are discussed further in Chapter ▶ 31.
Intraparenchymal injury is distinguished from subcapsular hematoma formation and injury to the splenic capsule. In doubtful cases with unexplained ascites and a suspected splenic rupture, ultrasound-guided fluid aspiration can be performed to confirm the hemorrhage. Abscess formation is observed in rare cases (for treatment see Chapter ▶ 15).
23.2.2 Splenic Infarction
Splenic infarctions usually resolve completely without sequelae or by scarring of the infarcted area. They most often occur in association with endocarditis, myeloproliferative syndromes, and septic diseases and generally do not require an ultrasound-guided intervention.1
23.2.3 Focal Splenic Changes
Cystic Masses
Dysontogenetic cysts appear echo-free with smooth margins, may contain internal septa, and display typical cystic features such as an entry echo and distal acoustic enhancement. Echogenic splenic cysts are more common than in the liver and require interventional investigation only in selected cases. Solitary echinococcal cysts are very rare; their management is discussed under PAIR treatment (puncture, aspiration, injection, reaspiration) for hydatid liver cysts in Chapter ▶ 17.9
Primary Splenic Tumors
Benign tumors of the spleen are relatively rare. As in the liver, the most common entities are capillary hemangiomas, which are usually echogenic; cavernous hemangiomas, which are hypoechoic or show variable echogenicity; and splenomas. Hemangiomas require differentiation from other mesenchymal tumors and littoral cell angioma. Only histology can furnish a definitive diagnosis. Benign/malignant differentiation in most cases relies on the progression of sonographic (imaging) findings. Primary malignant tumors of the spleen are extremely rare; most are mesenchymal tumors (angiosarcoma, leiomyofibrosarcoma).
Secondary Focal Splenic Changes
These changes are usually due to secondary infiltration by lymphoma, which may determine the individual prognosis (stage I or II [with primary mediastinal involvement] versus stage IV with splenic involvement). Lymphomatous infiltrates usually appear as hyperechoic, circumscribed lesions of variable size and with variable margins. They are distinguishable from a number of other changes (bacterial and fungal abscesses) only by histologic examination. Indolent non-Hodgkin lymphomas tend to show a diffuse or micronodular pattern of involvement, while more aggressive lymphomas may form conglomerate masses of variable size with mixed echogenicity and scalloped margins.
Splenic Abscesses
A distinction is made between macroabscesses and microabscesses (<10 mm). Splenic abscesses usually exhibit low or mixed echogenicity and are rarely echo-free. An echogenic perifocal reaction may be seen, depending on host immune status. Macroabscesses in the spleen most commonly result from the secondary infection of an infarcted area, the spread of pancreatitis, or hematogenous spread. The early stages of an abscess (pyogenic inflammation) may show increased vascularity. Microabscesses are typical of Candida infection. Multiple small focal lesions are found in immunosuppressed patients or patients who have had neutropenic fever. Hepatosplenic candidiasis is not manifested during neutropenic fever, and imaging findings appear only after recovery of the granulocyte count. For this reason, ultrasound is of little help in excluding HLC during the cytopenic phase.
Abscess regression in response to antimycotic therapy can be monitored sonographically. The differential diagnosis is broad and includes bacterial and fungal abscess, parasitic diseases, granulomatous inflammations, as well as lymphoma and hemophagocytic syndrome.
Isolated microabscesses <10 mm may determine the prognosis in any given case and are confirmed histologically (recommended needle diameter: 0.95 mm or 1.2 mm). Abscesses up to 50 (or 70) mm in size can be drained by percutaneous needle aspiration, while larger abscesses require catheter drainage as described in Chapter ▶ 15.
Splenic Metastases
Splenic metastases at the end stage of an aggressive cancer illness are more common than is generally assumed but rarely determine the prognosis. Splenic metastases are often hypoechoic, but some have a cystic appearance with echogenic elements while others show complex and variable echogenicity. Hypervascular metastases are found in association with neuroendocrine tumors and renal cell carcinoma, while relatively hypovascular metastases are more characteristic of gastrointestinal cancers.
23.3 Procedures
23.3.1 Clinical Scenarios
Splenic changes are often found in the setting of other organ manifestations. Splenomegaly seldom occurs in isolation (portal hypertension, infections, and hemato-oncologic diseases). Circumscribed splenic changes in otherwise healthy, asymptomatic patients generally have a benign etiology and require only follow-up.
Diagnostic and therapeutic interventions (drainage) are performed under primary sonographic guidance owing to the advantages described earlier. The indications and procedures are not standardized (ultrasound-guided splenic biopsy, laparoscopy, [exploratory] laparotomy [splenectomy], or wait and see). The indication for fine needle aspiration biopsy (FNAB) should be carefully considered in terms of the risk of hemorrhage and the alternatives of diagnostic and perhaps therapeutic splenectomy. The diagnostic accuracy of FNAB is high, however, and the postinterventional complication rate is low.
23.3.2 Anatomical Considerations in Splenic Interventions
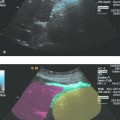
The anatomical relationships of the spleen should be considered prior to splenic interventions. The spleen is intraperitoneal, occupying a subdiaphragmatic and subcostal location in the left upper quadrant of the abdomen. The upper pole of the spleen is closely related to the gastric fundus, aorta, pancreatic tail, adrenal gland, and left lobe of the liver. It is relevant to interventions that, with deep inspiration, the spleen may be covered by air in the costophrenic angle. The lower pole of the spleen is anterolateral to the left kidney and borders on the left colic flexure. The splenic artery runs cranial to the pancreatic body and along the pancreatic tail to the splenic hilum. The spleen can be identified in an initial flank scan above the left kidney, and an intercostal scan aimed at the splenic hilum should then demonstrate the spleen without artifacts (the patient’s left arm is raised to open up the intercostal space). The splenic vein and pancreatic tail should also be visible. The beam is directed anteromedially to display the stomach and inferolaterally to display the left kidney.
23.3.3 Procedures for Specific Applications
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree