Pediatric spinal arteriovenous shunts are rare and, in contrast to those in adults, are often congenital or associated with underlying genetic disorders. These are thought to be a more severe and complete phenotypic spectrum of all spinal arteriovenous shunts seen in the overall spinal shunt population. The pediatric presentation thus accounts for its association with significant morbidity and, in general, a more challenging treatment process compared with the adult presentation.
Key points
- •
Spinal arteriovenous shunts are rare.
- •
Many spinal arteriovenous shunts are associated with congenital conditions (eg, Cobb syndrome).
- •
Only symptomatic lesions should be treated.
- •
Spinal arteriovenous shunts are difficult to treat.
Introduction
Pediatric spinal arteriovenous shunts (PSAVS) are rare and, in contrast to those in adults, are often congenital or associated with underlying genetic disorders. They are thought to represent a more severe and complete phenotypic spectrum of all spinal arteriovenous shunts seen in the overall spinal shunt population. The pediatric presentation thus accounts for its association with significant morbidity and, in general, a more challenging treatment process compared with the adult presentation.
Approximately 10% of all central nervous system arteriovenous shunts are spinal, but of those, a much smaller proportion is thought to present in the pediatric age group. The proportion of pediatric patients presenting with this disease seems to vary widely in the published literature, and the incidence is generally underestimated because of the variable clinical presentation and difficulty in establishing the diagnosis. In one series of spinal arteriovenous shunts, pediatric patients accounted for approximately 20% of the population.
Introduction
Pediatric spinal arteriovenous shunts (PSAVS) are rare and, in contrast to those in adults, are often congenital or associated with underlying genetic disorders. They are thought to represent a more severe and complete phenotypic spectrum of all spinal arteriovenous shunts seen in the overall spinal shunt population. The pediatric presentation thus accounts for its association with significant morbidity and, in general, a more challenging treatment process compared with the adult presentation.
Approximately 10% of all central nervous system arteriovenous shunts are spinal, but of those, a much smaller proportion is thought to present in the pediatric age group. The proportion of pediatric patients presenting with this disease seems to vary widely in the published literature, and the incidence is generally underestimated because of the variable clinical presentation and difficulty in establishing the diagnosis. In one series of spinal arteriovenous shunts, pediatric patients accounted for approximately 20% of the population.
Classification of PSAVS
Several classification systems have been used historically, which were largely inconsistent and imprecise, focusing primarily on being descriptive. Mulliken and Glowacki proposed the first classification system for vascular malformations based on endothelial cell characteristics and thus the vascular biology of these lesions. Since then, more recent classification systems have attempted to incorporate the vascular biology of these malformations, because it leads to a better understanding of the character and natural history of these lesions. This classification ultimately aids the neurovascular interventionalist in determining endovascular management.
One of the more recently proposed classification systems, based on 25-year experience at the Bicêtre Hospital, begins with considering underlying hereditary conditions, which provide some evidence for a genetic role in the development of PSAVS. In a series of patients younger than 2 years, 62% with PSAVS demonstrated either hereditary hemorrhagic telangiectasia (HHT) or a somatic nonheritable mutation/spinal arteriovenous metameric syndrome (SAMS). HHT is an autosomal dominant disease with identified mutations in 3 genes, 2 of which are members of the transforming growth factor β (TGF-β) pathway, which plays an important role during the embryologic development of vascular assembly and angiogenesis. Genetic nonhereditary conditions, such as Klippel-Trenaunay syndrome, Parkes Weber syndrome, neurofibromatosis I, and Ehlers-Danlos syndrome type IV, have also been associated with PSAVS.
The morphology of the shunt is then determined and classified into either a fistula, consisting of a single direct point of arteriovenous shunting, or a nidus, whereby the arteriovenous shunt occurs through a network of vessels. Fistulas are further classified into either microfistulas or macrofistulas. Micro–arteriovenous fistulas are small-size lesions fed by one or multiple arteries of different caliber, which drain into moderately enlarged veins, whereas macro–arteriovenous fistulas are high-flow shunts supplied by large arteries, which drain directly into a giant varix with secondary congestion of spinal cord veins.
The distinction between a fistula and nidus can be difficult to determine even if characterization via catheter angiography is performed, because arteriovenous shunts can induce progressive vascular changes over time with more prominent venous ectasia and recruitment of further arterial supply. Thus a single fistulous point may appear as a multiply shunting process resembling a nidus with time because of the pattern of pial reflex and congestion.
The compartmentalization of the shunt is the final consideration; this is classified into pial (intradural), dural (epidural), or paraspinal (parachordal). The pediatric dural shunts described here are not to be confused with and are distinct from the adult spinal dural arteriovenous fistulas, which are acquired lesions.
In using this classification system, it becomes apparent that the character of the shunt and its location are the most important factors in determining the management options and thus the likely outcome of subsequent endovascular treatment.
Clinical presentation
Although the angioarchitecture of PSAVS is an important determinant of treatment and its outcome, no definite evidence suggests that it determines clinical presentation, particularly hemorrhage, in these patients. Similarly, considering the strong association with hereditary conditions such as HHT and SAMS, very few patients presenting with PSAVS fulfill the clinical diagnostic criteria for HHT or have stigmata of a metameric syndrome.
A series of patients with intradural PSAVS showed that 19% presented at age 15 years and younger, with a high male predominance in contrast to the gender parity in the adult population. Most of the shunts in this series were in the cervical and thoracic spinal regions, with twice as many nidal-type shunts than fistulas. Hemorrhage at presentation was seen in 21 of the 30 patients: 11 with hematomyelia and 10 with subarachnoid hemorrhage. Patients with hematomyelia had severe deficits at presentation compared with their counterparts with subarachnoid hemorrhage. The remainder of the patients who presented without hemorrhage had acute symptoms attributable to venous congestion with or without venous thrombosis.
In another series in patients younger than 2 years, the proportion presenting with hemorrhage was much smaller, approximately 23% overall, but around 20% of those with intradural PSAVS. The mean age of presentation in that series was 7 months, with a similar male predominance to that seen in older children.
Diagnostic imaging
Magnetic Resonance Imaging
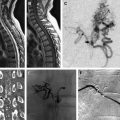
Magnetic resonance imaging (MRI) is the preferred modality for screening for PSAVS, and often requires imaging of the entire spinal axis. Computed tomography (CT) and conventional/CT myelography have little role to play the diagnosis and premanagement evaluation of spinal arteriovenous shunts.
Conspicuous and tortuous flow voids representing congested dilated veins are detectable on standard spin-echo, high-resolution steady-state Constructive Interference in Steady State (CISS)/Fast Imaging Employing Steady State Acquisition (FIESTA), and high-resolution 3-dimensional fast spin-echo with variable excitation pulse Sampling Perfection with Application optimized Contrasts using different flip angle Evolution (SPACE) sequences. The venous dilatation can often elude the site of the shunt in relation to the dura. Current contrast-enhanced time-resolved magnetic resonance angiography techniques are similarly useful in detecting the presence of a shunt, but currently little evidence shows its role in reliably identifying the site of arteriovenous shunting, although it can often visualize the location of an arteriovenous malformation (AVM) and the approximate location of its implied arterial feeders.
MRI is also useful in visualizing flow-related aneurysms, associated compression, edema, and hemorrhage affecting the spinal cord. Gadolinium contrast enhancement is sometimes seen, attributed to venous stasis, ischemia, or infarction of the spinal cord. In cases of SAMS, lesions of the associated metamere can often be seen. The utility of MRI is limited, however, in distinguishing between a nidus and an arteriovenous fistula and detecting small ventral shunts with draining veins within the ventral sulcus of the spinal cord.
Spinal Angiography
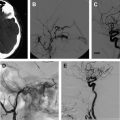
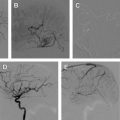
Currently, spinal angiography is considered the gold standard imaging modality for PSAVS. It is an invasive procedure performed with the patient under general anesthesia, requiring experienced neurointerventional expertise. Selective injections of all feeding pedicles and analysis of the draining veins is essential in the assessment and understanding of the angioarchitecture of PSAVS. This procedure also includes the assessment of the vascularity of adjacent spinal cord, which is crucial for preembolization treatment planning.
However, the intradural spinal arteries in neonates and infants are proportionately larger and more tortuous than in the normal adult, and therefore should not be misinterpreted as a pathologic finding.
Three-dimensional rotational spinal digital subtraction angiography (DSA) can be helpful in characterizing the PSAVS, particularly in identifying intranidal pseudoaneurysms and evaluating the feasibility of embolization.
Therapeutic strategy
The therapeutic strategy is determined on an individual basis relating to the expected clinical objective, be it preventive, corrective, or palliative. The choice of either primary neurosurgical treatment, with or without preembolization, or primary endovascular treatment will differ depending on the individual case and the institution. The results of either vary accordingly in the published literature.
The treatment approach and likely outcome is dependent on the angioarchitecture of the PSAVS. Fistulas have a greater likelihood of complete cure than AVMs, because the latter has a more complex anatomy. In AVMs this often precludes complete embolization, which, if attempted, may raise the risk of permanent neurologic deficit; partial targeted embolization of AVMs thus offers an improvement without unduly increasing this risk.
Treatment of PSAVS should almost never be on an emergent basis. Careful diagnostic angiographic assessment of the angioarchitecture of the shunt and determination of a preembolization strategy should be advocated before embarking on treatment.
Patients presenting with hemorrhage are supportively managed initially to allow recovery before any intervention is performed with embolization, usually 6 to 8 weeks after the initial event. Early or frequent rehemorrhage is not a typical feature in PSAVS, although differences may exist in extremely young patients. If an arterial pseudoaneurysm is identified in association with a ruptured PSAVS, partial targeted embolization may be considered, similar to the approach for ruptured brain AVMs.
The choice of delivery catheters and embolic material, and the choice of arterial, venous, or combination approaches can be variable. The methods outlined in the following sections reflect the treatment choice and strategy developed at the authors’ institution, elements of which are common to other institutions but not prescriptive.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree