Spinal Infection and Inflammatory Disorders
Renato Adam Mendonça
Introduction
This chapter discusses the role of magnetic resonance imaging (MRI) in the diagnosis of infectious and other inflammatory conditions that affect the spine and its contents. The most common of these conditions will be discussed according to their location, using an external to internal anatomic approach. Subsequently, many of the infectious pathogenic agents are discussed individually.
Infectious Spondylodiscitis
Infectious spondylitis is a condition that may affect one or more of the components of the spinal column and can be described etiologically as pyogenic, granulomatous (tuberculous, brucellar, fungal), and parasitic. Although rare, it is the main manifestation of hematogenous osteomyelitis in patients aged over 50 years (1) and represents 3% to 5% of all cases of osteomyelitis (1). The incidence of pyogenic spondylodiscitis is around 1:250,000 (1). Hematogenous spread, resulting in lodgment of organisms in the vertebral marrow, is the accepted mechanism of spondylodiscitis in adults. Although the venous plexus of Batson and arterial systems have both been implicated in carrying the pathogens, the latter is now recognized as the more important path to infection.
In order to distinguish the different patterns of disease in adults and children, it is important to have some understanding of the vascular anatomy of the spine and its developmental modifications. In children, intraosseous arteries display extensive anastomoses that prevent a septic embolus to produce a substantial osseous infarct. On the other hand, as terminal arterioles penetrate the disc, infection affects initially and is usually limited to this structure. In adults, the disc is avascular, the intraosseous anastomoses involute, and the intraosseous metaphyseal arteries become end vessels, for this reason susceptible to infarcts. A septic embolus can then cause a septic infarct of a large wedge-shaped subdiscal area of bone when it occludes one of them. Due to the relatively slow flow of the intraosseous metaphyseal artery, the thrombus will eventually extend retrogradely and circumferentially, obtruding the origins of other intraosseous metaphyseal arteries, disseminating the process to the whole plateau.
Further progression of the infection is also dependent on the vascular anatomy. As the adult superficial net of arteries become extensive, the infection can extend straight to the opposite vertebral endplate of the same vertebral body, without affecting its central part, through primary periosteal arteries. It can also reach the adjacent vertebral body endplate, across an intact disc, through intermetaphyseal arteries.
Pyogenic spondylitis is most frequently caused by hematogenous spread from distant infectious foci, but it can occur also by direct inoculation, most commonly iatrogenic following spinal surgery, lumbar puncture or epidural procedures, or by contiguous spread from neighboring infected organs like the oropharynx, pleural cavity, and thoracoabdominal wall. Rarely, it may follow stab or gunshot wounds to the spine.
The most common sources of septic emboli are, in decreasing order of frequency, infections from genitourinary tract, skin, and upper respiratory tract. Staphylococcus aureus is the most common organism identified (up to 60%), 2% to 16% of which are reported to be methicillin-resistant S. aureus (MRSA) (1) followed by Enterobacteriaceae (up to 30%). Other organisms commonly isolated include Staphylococcus epidermidis, Haemophilus influenzae, and different groups of Streptococcus. Salmonella infection is known to be more common in patients with sickle-cell disease.
Elderly diabetic patients, 60 to 70 years old, are more frequently affected, men almost twice than women. Hematogenous pyogenic spondylodiscitis affects preferentially the lumbar spine, followed by the thoracic and cervical spine in decreasing frequency (58%, 30%, and 11% respectively) (2), possibly reflecting the relative proportions of blood flow.
Other risk factors include advanced age, injecting drug use, immunosuppression, malignancy, renal failure, rheumatologic disease, liver cirrhosis, and previous spinal surgery (Table 24.1).
Spondylodiscitis is characterized clinically by back pain, localized tenderness, muscular spasm, and stiffness with or without neurologic compromise. The presence of fever is variable, and if absent should not sway anyone away from the diagnosis. The time interval between onset of suggestive clinical symptoms and presentation to medical services is between 2 weeks and 6 months, most often between 2 and 6 weeks. The erythrocyte
sedimentation rate (ESR) is almost always elevated and constitutes a good laboratory index. C-reactive protein (CRP) is similarly raised in the large majority of cases with spondylodiscitis and some authors suggest that it is the preferred marker for monitoring response to treatment (3). The leucocyte count is the least useful among the inflammatory markers; it is high in only a third to half of affected patients.
sedimentation rate (ESR) is almost always elevated and constitutes a good laboratory index. C-reactive protein (CRP) is similarly raised in the large majority of cases with spondylodiscitis and some authors suggest that it is the preferred marker for monitoring response to treatment (3). The leucocyte count is the least useful among the inflammatory markers; it is high in only a third to half of affected patients.
TABLE 24.1 Predisposing Factors for Spinal Infections (2) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Although the final diagnosis relies on culture of causative organisms from a biopsy sample, blood culture, pus culture, or even urine culture, it is not infrequent to treat patients without identification of the agent. Cultures of specimens may be falsely negative in up to 39% of cases of osteomyelitis (3). The accuracy of histopathology in the diagnosis of osteomyelitis is high, but its clinical utility is limited since the antimicrobial treatment must be guided by the identification of the causative organism. Besides that, MRI can detect and diagnose the infection noninvasively.
The primary treatment of the neurologically intact patient involves the use of immobilization and antibiotics (3). In most cases this is effective, and surgical intervention is not required. However, in a small proportion of cases, open or endoscopic surgery is warranted. Radiographic and MR findings may be very slow or inconsistent in resolution of infection-related abnormalities. For this reason the efficacy of conservative treatment may be estimated in individual cases by diminution of pain, resolution of fever and leukocytosis, as well as by a declining ESR and CRP. A falling ESR during the first month of nonsurgical treatment is a good prognostic sign; however, success of conservative treatment is seen in 40% of cases with persistently elevated or rising ESR.
Plain radiographies have a sensitivity of 82%, specificity of 57%, and accuracy of 73%, and they are frequently employed as a screening test. They may reveal early changes such as subchondral radiolucency, loss of definition of the endplate, and loss of disc height. Later changes include destruction of the opposite endplate, loss of vertebral height and paravertebral soft tissue mass. The radiologic changes, however, tend to appear only 2 to 8 weeks after onset of symptoms and false positive results can occur due to degenerative changes.
Several tracers have been used in the radionuclide imaging of spondylodiscitis. Technetium-99 m-methylene diphosphonate bone scintigraphy has a reported sensitivity of 90%, but a poorer specificity of 78%, degenerative changes resulting in false-positive results. Gallium-67 scintigraphy is a valuable adjunct to bone scan and when combined they have a sensitivity of 90%, a specificity of 100%, and accuracy of 94% (Table 24.2). Fluorine-18 fluorodeoxyglucose positron emission tomography (FDG-PET) can effectively distinguish infection from degenerative changes even when MRI is inconclusive, although it cannot differentiate infection from neoplasm.
TABLE 24.2 Diagnostic Studies in Spinal Osteomyelitis | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Computed tomography (CT) is the best modality at delineating bony abnormalities, including early endplate destruction before they become visible on x-ray, later sequestra or involucra formation, or pathologic calcification suggestive of tuberculosis (TB). It is inferior to MRI in imaging neural tissue and abscesses. Disc changes appear as hypodense areas. CT is currently mostly used for the radiologic guidance of spinal biopsy.
MRI is unquestionably considered the modality of choice for the radiologic diagnosis of spondylodiscitis. It has a reported sensitivity of 96%, specificity of 93%, and accuracy of 94%, with the advantage of providing anatomical information related to the paravertebral muscles, the epidural space, and the spinal cord.
In spite of MRI being the most sensitive technique for diagnosing spondylodiscitis, its findings may still lag behind the clinical symptoms, which may even include severe back pain. When the diagnosis is uncertain, a follow-up MR in 1 week may be helpful to show the evolution of the early changes.
The MR findings of spondylodiscitis include the following:
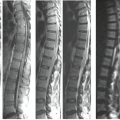
Areas of low signal intensity on T1-weighted images (T1WI) and high signal intensity on T2-weighted images (T2WI) (Figs. 24.1 and 24.2), the latter better depicted on fat-saturated, fast spin-echo (FSE) or short-TI inversion recovery (STIR) images). They are usually homogeneously distributed throughout the whole vertebra but mostly at one of the vertebral body metaphyses. These signal alterations often precede the destructive changes and are predominantly a consequence of edema.
The intervertebral disc presents variable intensity on T2WI, typically hyperintensity, and one cannot identify the internuclear cleft. The height of the intervertebral disc may be reduced (Figs. 24.2 and 24.3). Sometimes disc material can herniate into the softened neighboring endplates (Fig. 24.2) or into the spinal canal.
There is discontinuity of the margin between the disc and the intervertebral body, better depicted on T2WI (Figs. 24.1B and 24.2B).
Diffuse and homogeneous enhancement is seen in the affected marrow and most of the infected discs (Figs. 24.1C and 24.2C) (4).
Extension of the process into the paraspinal region is variable, being insignificant in most cases of pyogenic spondylitis. Some granulation tissue, however, may be produced and distributed evenly and circumferentially around the vertebral body (Fig. 24.4C). Intravenous administration of paramagnetic agents has been shown to be very important to establish with confidence the presence of epidural extension and associated meningeal inflammation. When there is a true abscess, the enhancement is peripheral, helping characterization and delimitation.
Intraosseous or extra-axial abscesses show restricted diffusion (bright) on diffusion-weighted imaging (DWI) and dark appearance on diffusion-weighted maps. DWI sequence may help to distinguish acute from chronic stage of the disease. These findings are summarized in Table 24.3.
In the same way in which the MR findings lag behind the early signs of disc space infection, they also lag behind in the healing phase of vertebral osteomyelitis. Once adequate antibiotic treatment has been instituted, the clinical symptoms improve dramatically, whereas the MR findings evolve much more slowly. The findings of healing osteomyelitis include persistent disc space narrowing, decreased signal intensity of the disc on T2WI consistent with disc degeneration, fusion of the adjacent vertebral bodies, and resolution of the high signal intensity in the adjacent endplates corresponding to resolution of the edema (Fig. 24.3). If there was an epidural or paraspinal abscess, these compartments also return to normal. In the early stages of treatment, laboratory findings such as sedimentation rate, CRP, and white cell count are more helpful in monitoring the response to treatment than the MR findings. Follow-up in patients with spondylodiscitis after the first 2 months can easily be done with MR. The finding of high signal intensity on T1WI from a previously infected vertebra reflects replacement of cellular marrow by fat, indicating healing.
Involvement of posterior elements of the spine with infection can be isolated to the facet joint and pedicle or lamina or can be contiguous with anterior vertebral body infection, which occurs more commonly with tuberculosis than with pyogenic spondylytis.
Childhood discitis is an infectious process of the intervertebral disc that differs from that of the adult because of patterns of arterial spine circulation. In children there are terminal arteries to the disk and consequently it is affected primarily by the germ, while in teenagers and in adults the disc involvement follows that of the endplate. It has a bimodal distribution occurring between 6 months and 4 years of age, with a second peak from 10 to 14 years of age. It most commonly involves the lumbar region at the L2–L3 and L3–L4 levels.
Clinical presentation is variable and there is frequently a delay in the diagnosis due to the uncharacteristic symptoms and signs as well as to the difficult communication with child of 1 to 3 years of age. Symptoms include fever, irritability, refusal to walk, back pain, inability to flex the lower back, and a loss of lumbar lordosis. Mild leukocytosis and an elevated ESR and CRP levels are usually present; results of blood cultures can often be negative. In one-third to one-half of patients, however, results of blood cultures or biopsy materials are positive and the infectious agent is almost always S. aureus.
Radiographs of the spine are usually normal in the early stages of disease, findings of bone scintigraphy can be positive as soon as 1 to 2 days after the onset of symptoms, demonstrating increased uptake in the intervertebral bodies on each side of the disk involved. However, bone scintigraphy is not specific and cannot differentiate discitis from other causes of back pain. Radiographs become very specific after the second and third weeks of disease. In a series of 33 children with discitis, 76% had abnormalities detected on spine radiographs and the most frequent finding was decreased height of the discs and erosion of adjacent vertebral endplates.
MRI is the study of choice because it can detect discitis early on, reduces the diagnostic delay, and may help to avoid the requirement for a biopsy. MRI findings include loss of the normal hyperintense signal intensity of the disk on T2WI, narrowing or complete absence of the disc, and abnormal increased T2WI in the adjacent vertebral bodies, consistent with marrow edema. There may be contrast enhancement of the disk and adjacent vertebral body, disc herniation, extradural flegmon, and abscess. The evaluation for suspected discitis must exclude spinal cord compression (5).
Treatment includes bed rest, spinal immobilization, and most children receive systemic antibiotics, what is usually successful. Some argue that the natural history of this condition is benign and that antibiotics are only indicated when symptomatic treatment, such as immobilization, has failed.
Follow-up radiographs will show persistent narrowing of the intervertebral disk space and sclerosis at adjacent vertebral bodies weeks or months after the initial diagnosis. Most patients will be asymptomatic within 3 weeks following antibiotic treatment. Disc space height can sometimes be restored. Patients
must be followed up at least for 2 years, when most spines are mobile and the patients free from pain. Radiologic fusion occurs in approximately a fifth of the cases. MRI shows variable appearances: changes in the vertebral body usually resolve at 24 months and recovery of disc is seen after 34 months (5).
must be followed up at least for 2 years, when most spines are mobile and the patients free from pain. Radiologic fusion occurs in approximately a fifth of the cases. MRI shows variable appearances: changes in the vertebral body usually resolve at 24 months and recovery of disc is seen after 34 months (5).
Discitis should be considered as diagnosis in children with refusal to walk or gait disturbances especially in combination with elevated ESR. MRI is the tool of choice to set the diagnosis early. With an adequate and early therapy with bracing and antibiotics, a result without spine instability or deformity can be achieved.
Postoperative spondylodiscitis is an infrequent complication of lumbar disc surgery, being the reported incidence 1%-3, 4% (6). The most probable cause is intraoperative contamination rather than hematogenous spread, although both may happen in a surgically traumatized and poorly vascularized area (locus minoris resistentiae). The most common clinical features are recurrent pain after initial postoperative relief, muscle spasm, elevated temperature, and positive straight leg-raising test. ESR is not a good test because it is usually increased postoperatively in the absence of infection. CRP is a more reliable screening test for infections after lumbar disc surgery, especially if it was known to be negative before surgery. The roentgenographic findings appear several weeks after the initial symptoms. MR may be helpful earlier, but only infrequently it is possible to reliably diagnose infection before 3 weeks. Distinguishing early MR findings of postoperative discitis from normal postoperative disc space changes may be a challenge. Depending on the surgical technique, the operated disc and the adjacent endplates may show more or less extensive
changes, besides those existing previously to the surgery. These changes are due to the intervention itself and to the degree of supervening aseptic inflammatory response. A minimally invasive surgery of the intervertebral disc is completely different from extensive disc instrumentation for placement of a cage, for example, making sometimes impossible the differential diagnosis with infection.
changes, besides those existing previously to the surgery. These changes are due to the intervention itself and to the degree of supervening aseptic inflammatory response. A minimally invasive surgery of the intervertebral disc is completely different from extensive disc instrumentation for placement of a cage, for example, making sometimes impossible the differential diagnosis with infection.
Gadolinium enhancement of the vertebral bone marrow, disc space, and posterior annulus fibrosus is not specific for bacterial infection, being seen also in asymptomatic patients what makes MRI more effective for exclusion than for confirmation of postoperative spondylodiscitis. Suspicion of septic postoperative discitis should be confirmed by MRI, serum CRP, and disc puncture. MRI is not reliable as the sole method for distinguishing septic from aseptic discitis in the early postoperative stage (6). Some general guidelines, however, may be followed:
The absence of vertebral endplate changes with low signal on T1WI and high signal on T2WI makes septic spondylodiscitis highly unlikely.
The absence of contrast enhancement of the intervertebral space makes spondylodiscitis improbable.
TABLE 24.3 Magnetic Resonance Imaging of Spondylodiscitis
Near endplates or near whole vertebrae: low intensity on T1-weighted imaging and high intensity on T2-weighted imaging
High intensity and loss of the internuclear cleft on T2-weighted imaging
Loss of a margin between disc and vertebral bodies
Irregularities and destruction of the vertebral endplates, eventually followed by destruction of the whole vertebral body
Enhancement following magnetic resonance imaging signal changes; annular enhancement indicates abscess formation
Osseous and extra-axial abscesses show restricted diffusion on diffusion-weighted imaging and dark appearance on diffusion-weighted maps
An enhancing rim of soft tissue around the affected intervertebral space is suggestive of spondylodiscitis.
The presence of two parallel thin bands of enhancement in the disc space suggests postdiscectomy, while more amorphous enhancement is generally seen with infection.
Conservative management based on bed rest, immobilization, and antibiotics is the initial treatment of choice for postoperative spondyldiscitis. However, surgical treatment is indicated much more frequently than in cases of spontaneous septic spondylodiscitis.
The differential diagnosis of septic spondylodiscitis includes degenerative changes, granulomatos spondylitis, dialysis-related arthropathy (Fig. 24.5), pseudoarthrosis, neuropathic arthropathy, and Richter syndrome (Table 24.4).
Hematogenous Pyogenic Facet Joint Infection
This is a rare but underdiagnosed condition that occurs primarily in the lumbar spine (Fig. 24.6) but also in the cervical spine and rarely in the thoracic spine (Fig. 24.7). The exact incidence of this entity is unknown because it is believed that some of the patients may heal spontaneously without any treatment. Although pyogenic facet joint infections may occur after facet joint injections or complicating an epidural abscess, most cases are primarily hematogenous. As part of the spine pyogenic infection spectrum, the predisposing factors, the infectious agents, and probably the same physiopathology are the same as those previously mentioned for pyogenic spondylitis.
TABLE 24.4 Differential Diagnosis of Infectious Spondylodiscitis | |||||
|---|---|---|---|---|---|
|
It represents around 4% of all hematogenous spinal osteomyelitis and ESR and CRP are elevated in all cases. Positive tissue and/or blood cultures are easily obtained. Formation of epidural abscess complicates 25% of the cases; of these, 38% develop severe neurologic deficit. The usual presentation of lumbar facet joint infection is severe back pain that may radiate to the flank or buttocks and does not improve with bed rest.
Hematogenous cervical facet joint infection shares many characteristics with the more common lumbar entity. However, the very few cases reported have been complicated by epidural abscess or granulation tissue formation that has led to neurologic deficit, suggesting a less benign course than in the lumbar spine. When it occurs in the cervical spine there may be stiffness of the neck besides neck and trapezius muscle pain. Patients may be febrile, present chills, and report radicular symptoms. The time between initial symptoms and diagnosis is a little shorter than for spondylodiscitis, around 4 weeks. In addition, the symptoms associated with facet arthritis tend to be unilateral and present more acutely and severely in the earlier stages.
Imaging studies are essential for diagnosis. Plain radiographs, including oblique views, may show changes consistent with erosive arthritis of the interapophyseal joints after 6 to 12 weeks. These changes consist in narrowing or enlargement of the articular space and the presence of irregular lytic lesions and erosions on the facets themselves.
CT can show alterations earlier than that, although they may take as long as 9 weeks to appear: loss of subchondral bone adjacent to the facet joint, variable expansion of the joint with fluid density, loss of density of ligamentum flavum, presence of mixed lytic and sclerotic changes, and presence of contiguous posterior paraspinal phlegmon inflammation or abscess.
Although nonspecific, technetium-99 bone scintigraphy and gallium-67 scintigraphy are both sensitive in the early detection of septic facet join arthritis but may be falsely negative in the first week.
MRI is sensitive and specific in diagnosing pyogenic facet joint infection as early as 2 days after the beginning of symptoms. Besides being more sensitive, MR shows signal intensity changes and enhancement of the affected bone structures and soft issue component of the lesion (Figs. 24.6 and 24.7; Table 24.5). The differential diagnosis includes neoplastic processes, erosive arthritidis such as rheumatoid arthritis (RA), multicentric reticulohistiocytosis, and scleroderma.
TABLE 24.5 Magnetic Resonance Imaging of Septic Arthritis of Apophyseal Joints | |||||
|---|---|---|---|---|---|
|
Rheumatoid Arthritis
The cervical spine involvement in RA is frequent and early in the course of the disease. The positive radiographic signs of its involvement are in the range of 43% to 86% depending on the duration of the affection. After the metacarpophalangeal joints the cervical spine is the most common site affected in RA.
Acute and chronic manifestations of synovitis in patients with RA were characterized with MRI including contrast-enhanced images obtained immediately and 5 minutes after the injection, and histologically correlated, as follows:
Joint effusion: hypointense on T1WI, hyperintense on T2WI, enhancement initially on the periphery, homogeneous enhancement on late images
Hypervascular pannus: hypointense on T1WI, hyperintense on T2WI-weighted, intense, and early enhancement that remain constant on late images
Hypovascular pannus: hypointense signal on T1WI, intermediate signal on T2WI, moderate enhancement that persists o late images
Fibrous pannus: hypointense on T1WI and on T2WI, slight if any enhancement
The usual findings at the discovertebral junction of patients with RA are intervertebral disc space narrowing, subchondral osseous irregularities, and erosions with adjacent eburnation.
Osteophytes are not included, which constitutes an important element in the differential diagnosis with degenerative changes. In the interapophyseal joints, narrowing and erosions are common. In approximately 10% of patients with RA, erosions or even destruction of one or more spinous processes can be detected. Evolving RA can eventually lead, sometimes in only 2 years after its first clinical manifestation, to three different patterns of instability in decrescent frequency: atlantoaxial subluxation, subaxial subluxation, and atlantoaxial impaction.
Subaxial subluxations can be observed at one or more levels of the cervical spine caudad to C2, particularly at the C3–C4 and C4–C5 levels (Fig. 24.8).
The prevalence of atlantoaxial subluxation is between 12% and 33% (4,7), most of them anterior atlas axis subluxation (AAAS). Although it is more frequently asymptomatic, it can evolve initially to compression of the occipital nerve roots, and sequentially to compression of the spinal cord, resulting in myelopathy with long tract symptoms, tetraparesis and, without proper treatment, to death.
Atlantoaxial instability is a consequence of transverse ligament laxity that follows synovial inflammation between the dens and the atlas. It can be further accentuated by erosion of the odontoid process, detected in 14% to 35% of RA patients (Figs. 24.9 and 24.10). AAAS is diagnosed when the distance between the posteroinferior aspect of the anterior arch of C1 and the most anterior aspect of the dens (ADI) is ≥3 mm.
Dislocations can occur also in vertical and lateral directions. Vertical subluxation, atlantoaxial impaction, and cranial settling are names given to the upward migration of the odontoid process caused by loss of the supporting ligamentous structure. It is a relatively uncommon and life-threatening complication that affects 5% to 8% of patients with longstanding RA. In lateral subluxation of the atlantoaxial joints, the atlas shifts and tilts laterally due to bone erosion, disruption of the articular capsules, or collapse of the lateral masses of the axis.
Evaluation of the craniocervical junction is clinically difficult, which is why the examination is frequently supplemented with imaging diagnostic methods. The radiographic study can include the careful dynamic assessment with films obtained with the patient flexing and extending the neck to determine the existence of instability. Besides the dynamic evaluation, plain films can detect only advanced stages of the disease, and multichannel spiral CT is the preferred method for evaluating the effect on bone structures at the higher cervical spine. Evaluation of the soft tissues is still better achieved with MRI. Dynamic MRI studies of the cervical spine can also be obtained with the acquisition of sagittal images, respectively, with flexion and extension of the neck (Fig. 24.11). Some authors suggest that this procedure be realized in patients with RA in whom
cervical subluxation is suspected and routine MRI findings were equivocal. However, when dynamic studies are performed, patient monitoring is advised, and rapid sequences are desirable because the position is risky and uncomfortable. The procedure is unnecessary and even contraindicated in patients in whom medullary or spinal cord compression is discovered on studies made in the neutral position. It was recently shown that MR is more sensitive than standard dynamic studies of the cervical spine to diagnose AAAS. It provides important biomechanical clues, other than ADI, that improve accuracy in diagnosing atlantoaxial instability. These signs are the presence of significant amount of pannus, dental erosion, tilting of anterior atlantoaxial joint, peridental effusion, lateral facet arthropathy, abnormal spinolaminar line, and focal myelopathy. The combination of peridental effusion, lateral facet arthropathy, abnormal intramedullary signals, and abnormal spinolaminar line showed a sensitivity of 100% and a specificity of 90% in diagnosing AAA subluxation (8). The imaging evaluation of cranial settling deserves special consideration because of the erosion of the odontoid process. In this case, the Redlund–Johnel line is used, which measures the distance from the base of C2 to the plane of Chamberlain’s line. Cranial settling is considered present when the distance between the cortical margin of the base of the axis and the Chamberlain’s line is less than 29 mm in women or 34 mm in men. Approximately 6% of the cases of atlantoaxial impaction or cranial settling in rheumatoid patients would be missed even with elaborate plain radiographic studies. For this reason, CT or MRI should be performed on an RA patient whenever plain radiographs leave any doubt about the diagnosis of vertical subluxation. In addition, it is mandatory to use MRI of the cervical spine whenever there is suggestion of spinal cord compression.
cervical subluxation is suspected and routine MRI findings were equivocal. However, when dynamic studies are performed, patient monitoring is advised, and rapid sequences are desirable because the position is risky and uncomfortable. The procedure is unnecessary and even contraindicated in patients in whom medullary or spinal cord compression is discovered on studies made in the neutral position. It was recently shown that MR is more sensitive than standard dynamic studies of the cervical spine to diagnose AAAS. It provides important biomechanical clues, other than ADI, that improve accuracy in diagnosing atlantoaxial instability. These signs are the presence of significant amount of pannus, dental erosion, tilting of anterior atlantoaxial joint, peridental effusion, lateral facet arthropathy, abnormal spinolaminar line, and focal myelopathy. The combination of peridental effusion, lateral facet arthropathy, abnormal intramedullary signals, and abnormal spinolaminar line showed a sensitivity of 100% and a specificity of 90% in diagnosing AAA subluxation (8). The imaging evaluation of cranial settling deserves special consideration because of the erosion of the odontoid process. In this case, the Redlund–Johnel line is used, which measures the distance from the base of C2 to the plane of Chamberlain’s line. Cranial settling is considered present when the distance between the cortical margin of the base of the axis and the Chamberlain’s line is less than 29 mm in women or 34 mm in men. Approximately 6% of the cases of atlantoaxial impaction or cranial settling in rheumatoid patients would be missed even with elaborate plain radiographic studies. For this reason, CT or MRI should be performed on an RA patient whenever plain radiographs leave any doubt about the diagnosis of vertical subluxation. In addition, it is mandatory to use MRI of the cervical spine whenever there is suggestion of spinal cord compression.
Seronegative Spondyloarthritis
Seronegative spondyloarthritis is a chronic inflammatory rheumatic disease, seronegative for rheumatoid factor, and often associated with the presence of HLA-B27 (9). These diseases affect preferentially the axial skeleton, causing pain and stiffness by the preferential involvement of enthesis, but also of discs and synovial joints. In this group are included ankylosing spondylitis, by far the most common, reactive arthritis (former Reiter’s syndrome), psoriatic arthritis, arthritis associated with Crohn’s disease and ulcerative colitis, and undifferentiated spondyloarthritis.
CT and MRI are more sensitive and specific than conventional radiographs for assessing involvement of the spine and sacroiliac joint in these conditions (9), helping to optimize the treatment of patients. The most characteristic spinal involvement in spondyloarthritis is enthesitis, an inflammatory process affecting the insertions of the vertebral ligaments. MRI is best suited to depict the acute inflammatory enthesis damage and also the following fatty degeneration (9). CT shows better the more chronic sclerotic changes, and bone formation. One must remember, nevertheless, that all these alterations can occur simultaneously in the same patient, and that the chronic changes can also be shown by MRI, although less conspicuously.
Initial inflammatory phase histopathologic examinations show erosive lesions with infiltrating macrophages and lymphocytes at the insertion of ligaments. The adjacent marrow spaces depict edema, infiltration of plasma cells, and paucity of hematopoietic tissue (10). Four different manifestations can be identified: Romanus spondylitis at vertebral corners, Andersson aseptic spondylodiscitis, and arthritis of the facet and costal joints, all very similar to their pyogenic counterparts, as well as true ligamentous inflammatory involvement (9,10).
Romanus spondylitis describes the inflammatory changes involving the anterior and posterior edges of the vertebral endplates, secondary to enthesitis of, respectively, the anterior and posterior longitudinal ligaments. MRI can show the edematous corners hyperintense on T2WI, hypointense on T1WI, and enhancing after intravenous (i.v.) administration of gadolinium chelate.
Andersson aseptic spondylodiscitis encompasses the inflammatory changes involving the disc and adjacent vertebral endplates, which imaging aspect is similar to that described for pyogenic spondylitis. The disc and the endplates appear hyperintense on T2WI, hypointense on T1WI, all which may enhance after gadolinium administration. Bone erosions of the vertebral endplates may be observed later on CT.
Arthritis of the facet joint, costotransverse and costocostal joints acutely encompass bone marrow edema, effusion, erosions, and contrast enhancement, all best depicted by MRI. At the end stage, there are reactive subchondral bone formation, osseous fusion (Fig. 24.12), capsular ossification, and the articulations may undergo ankylosis, all better shown by CT or even in radiographs (9). At the atlantoaxial joints, inflammatory changes of the synovial and adjacent ligamentous structures can lead to erosion and resorption of the dens, similar to, but less frequent than, what is observed in RA.
In spite of the fact that ligamentous lesions are most commonly centered at the bone insertions, other parts of the ligament can be affected, corresponding to true ligamentous inflammation (9). Fat-saturated T1WI with administration of gadolinium is more sensitive than fat-suppressed T2WI sequences in the detection of this type of involvement. All vertebral ligaments may be affected, most often the interspinal and the supraspinal ligaments. Obviously, inflammation of the bone marrow adjacent to their insertions may also be seen (9).
Later in the course of the disease, inflammatory zones may be replaced by fatty bone marrow. MRI may show fatty infiltration at either edge of the vertebral endplates representing postinflammatory changes after Romanus spondylitis or at the vertebral margins associated with Andersson spondylodiscitis, the last mimicking Modic Type II degenerative changes. The final stage consists of sclerotic changes and formation of syndesmophytes through continuing enchondral ossification in most previously inflamed tissues, what eventually leads to ankylosis of the spine and of the sacroiliac joints. When syndesmophytes bridge several adjacent vertebral bodies, or even the
whole spine, the imaging of this structure resembles bamboo; hence the name “bamboo spine.”
whole spine, the imaging of this structure resembles bamboo; hence the name “bamboo spine.”
Calcification and ossification of the posterior longitudinal ligament, as well as of the interspinous and supraspinous ligaments, may be prominent in ankylosing spondylitis. Ossification of the posterior longitudinal ligament can cause compression of the spinal cord.
Insufficiency hyperextension vertebral fractures, known as Andersson fractures, may occur as a consequence of the ankylosis and osteoporotic changes (9). Because of the osteopenic changes, the fracture may pass through the vertebral body (transvertebral), but it may also pass through the disc space (transdiscal) or even through both. Several findings must be looked for in MRI of the spine to avoid missing this threatening condition (Table 24.6) (11).
Sacroiliac joints, predominantly made of fibrocartilage and containing very little synovial fluid, for these reasons considered enthesis, may be involved either unilaterally or bilaterally. The different stages of inflammatory sacroiliac involvement on CT and MRI follow the pattern previously described to the spine.
The earliest sign of sacroiliitis, inflammatory subchondral bone edema, is hyperintense on fat-saturated T2WI or STIR sequences and may enhance after administration of gadolinium chelates. Enhancement of the fibrous connective of the joints may also be present. CT may initially depict subchondral demineralization followed by bone erosions. Early diagnosis of either sacroiliac or spinal inflammatory involvement helps in initiating early treatment what may prevent ankylosis (9).
TABLE 24.6 Imaging Findings in Ankylosing Spondylitis Patients with Spinal Fractures or Pseudoarthrosis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Later in the course of the disease, inflammation usually decreases and subchondral edema is progressively replaced by fatty bone marrow, which appears hyperintense on T1WI. The final stage of sacroiliac involvement consists of subchondral sclerosis and fusion of the joint with ankylosis. At this stage, MRI may show sclerotic changes, hypointense on T1 and T2WI, and fusion of the articulation (9). However, in cases in which radiographs and MRI are equivocal, CT is the best imaging technique for depicting subchondral density and sacroiliac ankylosis (9).
The cauda equina syndrome (CES) is a late, rare, and poorly understood but well-recognized complication of longstanding ankylosing spondylitis. Symptoms of CES, which include cutaneous sensory impairment of lower limbs and perineum and sphincter disturbance, usually appear when ankylosing spondylitis is quiescent and laboratory tests are normal. Although the exact pathogenesis of CES is not known, the mechanisms of nerve root injury include arachnoiditis and compression from expanding thecal diverticula. There are cases in which the authors described the MR findings of florid multilocular dural ectasia, marked irregularity and thickening of nerves, and adherence to the diverticular in patients with ankylosing spondylitis and neurologic symptoms of CES. These cases provide evidence for the role of arachnoiditis in the pathogenesis of the CES of ankylosing spondylitis.
Gout
Gout is a common rheumatologic disease but is infrequently diagnosed in the axial skeleton, with fewer than 30 cases reported in the literature (12), what is probably underestimated because patients who may have asymptomatic tophi are not routinely imaged, and such lesions, even if diagnosed, are not pathognomonic. Most patients presenting spinal involvement by gout have chronic polyarticular tophaceous gout and hyperuricemia, with a mean duration of disease of 14 years. The clinical symptoms vary from none to acute quadriparesis. All segments of the spine are affected in approximately equal distribution. The frequency of neural compromise is high, leading frequently to an acute neurologic deficit that may require emergency surgical decompression.
A tophus is the pathognomonic lesion of gout, and patients usually have had gout for 10 to 12 years before these lesions become visible radiographically or on physical examination. It is a mass constituted by urates, either crystalline or amorphous,
surrounded by tissue showing increased vascularity and an intense inflammatory reaction composed of macrophages, lymphocytes, fibroblasts, and foreign-body giant cells. MRI data obtained from 13 patients showed all lesions to have T1 isointensity or hypointensity and variable signal intensity on T2WI that was related to the amount of calcium in the tofi (13). There is strong enhancement by gadolinium chelates (Fig. 24.13).
surrounded by tissue showing increased vascularity and an intense inflammatory reaction composed of macrophages, lymphocytes, fibroblasts, and foreign-body giant cells. MRI data obtained from 13 patients showed all lesions to have T1 isointensity or hypointensity and variable signal intensity on T2WI that was related to the amount of calcium in the tofi (13). There is strong enhancement by gadolinium chelates (Fig. 24.13).
Descriptions of the involvement of the spine by gout consist of extensive lytic lesions associated with a soft tissue mass, with the above-described characteristics, affecting more commonly the posterior vertebral structures. These lesions are sharply delineated, without surrounding infiltrative changes; normal disc tissue persists immediately adjacent to destroyed discal areas; and no significant bone marrow edema is seen in the trabecular bone adjacent to the lesion (12). It was recently shown a tophaceous gout hypermetabolism by FDG-PET (12), making the differential diagnosis with malignant tumors more difficult. It must be considered, however, that this diagnostic must be suspected on clinical grounds.
Spinal Epidural Abscess
Despite the progress in imaging, early diagnosis of spinal epidural abscess (SEA) remains difficult, and treatment is often delayed. The morbidity of SEA is high, and its mortality ranges from 18% to 31% in modern series (14). The predisposing factors and the etiologic agents of SEA are the same above described for pyogenic spondylodiscitis and pyogenic facet joints arthritis. S. aureus is the causative agent in 62% to 67% of cases, and in 15% of infections these organisms are methicillin resistant (14). The occurrence of SEA in children represents less than 1.5% of cases.
Prompt diagnosis and treatment are critical because SEA patients can rapidly progress to paraplegia, quadriplegia, and death if untreated or if treatment is delayed. The features suggesting the diagnosis are back pain, progressive neurologic deficit, low-grade fever, and obtundation; however, there may be no fever in the subacute and chronic cases. Peripheral leukocyte count is elevated in approximately 60% of patients, and ESR rate is elevated in most patients. The most frequent source of functional compromise of the spinal cord is mechanical compression (Fig. 24.14), but deterioration can be seen also related to ischemic compromise (Fig. 24.15).
It may be encountered in all segments of the spinal canal. SEA occurs most commonly in the lower thoracic and lumbar spine, followed by the cervical and upper thoracic spine. SEA can be classified as diffuse (Fig. 24.16) when it involves six or more vertebral segments and as focal when it involves five or
fewer vertebral segments (Fig. 24.17). The most common concomitant infections are spondylodiscitis, facet infection, posterior paraspinal abscess, and retroperitoneal abscess. Most cases are anterior to dural sac or involve it circumferentially and are associated with spondylodiscitis. When abscess involves the ventral epidural space, it tends to conform to a pattern anatomically dictated by the posterior longitudinal ligament/central septum complex and lateral membranes. MR is the most effective diagnostic technique for SEA, its sensitivity varying between 91% and 100% (Table 24.7).
fewer vertebral segments (Fig. 24.17). The most common concomitant infections are spondylodiscitis, facet infection, posterior paraspinal abscess, and retroperitoneal abscess. Most cases are anterior to dural sac or involve it circumferentially and are associated with spondylodiscitis. When abscess involves the ventral epidural space, it tends to conform to a pattern anatomically dictated by the posterior longitudinal ligament/central septum complex and lateral membranes. MR is the most effective diagnostic technique for SEA, its sensitivity varying between 91% and 100% (Table 24.7).
SEA can be hypointense, isointense, or slightly hyperintense compared with the spinal cord on T1WI, depending on its fluidity. When there is associated spondylodiscitis, the related vertebral bodies and intervertebral discs are hypointense on T1WI.
On T2WI, SEA is mostly hyperintense, being difficult to differentiate from cerebrospinal fluid (CSF) 3D FSE and 3D CISS, can help to overcome this difficulty, better showing the
SEA in relation to CSF and to the spinal cord. Postcontrast sequences can help to differentiate granulation tissue from frank pus, although this may not be clinically relevant because both conditions can result in neurologic compromise and require surgical decompression.
SEA in relation to CSF and to the spinal cord. Postcontrast sequences can help to differentiate granulation tissue from frank pus, although this may not be clinically relevant because both conditions can result in neurologic compromise and require surgical decompression.
TABLE 24.7 Concomitant Infectionsa in Patients with Spinal Epidural Abscess | |||||||
|---|---|---|---|---|---|---|---|
|
There are two main patterns of enhancement in SEA. The most common one is diffuse enhancement at the site of the solid component of the SEA, in either a homogeneous or heterogeneous fashion (Fig. 24.15). The second most common pattern of enhancement is a thin or thick rim around a collection of low signal intensity, representing, respectively, granulation tissue and pus (Figs. 24.16 and 24.17). Linear enhancement along the compressed dura mater may be observed in most patients with diffuse SEA on sagittal images (Fig. 24.16), which is not usually observed
in patients with focal SEA. Sagittal views are the most useful projections for assessment of cephalic and caudal extensions of SEA (Figs. 24.16A–C and 24.17A,B). Axial views are needed to define the exact site of granulation tissue and collections of pus relative to the dural sac and to the bony structures (Figs. 24.16D–F) as well as for demonstrating concomitant paraspinal abscess.
in patients with focal SEA. Sagittal views are the most useful projections for assessment of cephalic and caudal extensions of SEA (Figs. 24.16A–C and 24.17A,B). Axial views are needed to define the exact site of granulation tissue and collections of pus relative to the dural sac and to the bony structures (Figs. 24.16D–F) as well as for demonstrating concomitant paraspinal abscess.
To prevent serious morbidity and mortality, early diagnosis is essential for proper management of SEA. Patients at risk for developing such abscesses who present with local back pain and/or have an increased ESR and/or neurologic deficit should have an immediately MR scan, including postgadolinium sequences. Surgical drainage and prolonged antibiotic use are the cornerstones of treatment, although selected patients may be treated conservatively. Spinal DWI can better characterize epidural collections, as restricted diffusion, and should be part of the routine MRI of the spine (Fig. 24.18).
When an epidural abscess is primary, differential considerations include malignancy, particularly metastasis. Compared with neoplasms, more acute processes such as epidural abscess and hematoma more commonly violate the midline septum of the ventral epidural space (15). Epidural hematoma, extruded or migrated disc fragment, and epidural lipomatosis are included in the differential diagnosis too.
Spinal Hypertrophic Pachymeningitis
Hypertrophic pachymeningitis is a rare chronic inflammation of the cranial and/or spinal dura mater. The usual location of the hypertrophy is the cervical or thoracic spine, but the whole spine may be affected (16). Most cases of idiopathic pachymeningitis are characterized by a nonnecrotizing chronic inflammatory infiltrate of lymphocytes, plasma cells, and occasional histiocytes, giant cells, polymorphonuclear cells, or eosinophils. Granulomas, necrosis, and vasculitis are less frequently identified.
The etiology of hypertrophic pachymeningitis is unknown, but many causal factors or cofactors have been implicated, including Wegener’s granulomatosis, SLE, sarcoidosis, multifocal fibrosclerosis, orbital idiopathic inflammatory syndrome, RA, carcinomatosis, metabolic diseases, trauma, toxins, thrombophlebitis, syphilis, tuberculosis, fungi, HIV, HTLV-1, meningococcal meningitis, intrathecal steroid deposition, and vasculitis (16,17). For this reason, extensive workup is required to exclude all these causes in order to diagnose idiopathic hypertrophic pachymeningitis (Fig. 24.19). In the majority of cases, the final diagnosis is clear only after surgery (18,19).
Early surgical intervention can successfully improve neurologic symptoms. Laminectomy or laminoplasty followed by durotomy and duroplasty is the recommended surgical treatment for the disease (18,20,21). However, corticosteroids may achieve symptomatic control and reduction in dural thickness, which can be virtually complete.
It has been proposed that hypertrophic spinal pachymeningitis should be considered in the differential diagnosis for patients with spinal cord compression and radicular pain in more than three spinal levels (16). The clinical course of idiopathic hypertrophic spinal pachymeningitis (IHSP) may follow one of three patterns; sustained remission, relapse with corticosteroid resistance, or relapse with corticosteroid dependence (17). There are few studies regarding the frequency or cause of recurrence (19). An extended extramedullary mass of low T2 signal intensity with peripheral enhancement, linear or nodular, represents a specific MRI finding that is highly suggestive of IHSP. The linear enhancement pattern appears to show better therapeutic response than the nodular form, possibly related to less fibrosis and more vascularity. It is difficult on MRI alone, however, to distinguish these findings from diffuse leptomeningeal carcinomatosis.
Subdural Abscesses
Spinal subdural abscess (SSA) is very rare and its exact incidence is unknown; only less than 70 cases of patients with this condition
have been reported (22). S. aureus is the most frequent causative agent, the thoracolumbar region is the most frequent localization, and the group of risk is the same of spondylodiscitis, septic spondiloarthritis, and SEA. However, SSA is much less common than SEA and it is only infrequently related to spondylodiscitis. Most patient’s age are between 60 and 70 years (22,23). The development of SSA can be secondary to hematogenous spread of infection from other region, infected CSF and direct spread into the subdural space, hematogenous inoculation during the course of meningitis, secondary inoculation due to lumbar puncture, direct contact with intraspinal space (osteomyelitis), and secondary infection after spinal surgery (22). There are only two cases of SSA in the literature that are unrelated to such conditions and without well-documented etiology (24).
have been reported (22). S. aureus is the most frequent causative agent, the thoracolumbar region is the most frequent localization, and the group of risk is the same of spondylodiscitis, septic spondiloarthritis, and SEA. However, SSA is much less common than SEA and it is only infrequently related to spondylodiscitis. Most patient’s age are between 60 and 70 years (22,23). The development of SSA can be secondary to hematogenous spread of infection from other region, infected CSF and direct spread into the subdural space, hematogenous inoculation during the course of meningitis, secondary inoculation due to lumbar puncture, direct contact with intraspinal space (osteomyelitis), and secondary infection after spinal surgery (22). There are only two cases of SSA in the literature that are unrelated to such conditions and without well-documented etiology (24).
Back pain at the level of the affected spine, fever, and neurologic deficits such as para/tetraparesis, bladder dysfunction, disturbances of consciousness, and inflammatory signs are some typical symptoms of SSA (25).
Contrast-enhanced MRI is the imaging method of choice in this instance because it is less invasive and due to its superiority and sensitivity in detecting the exact location and extension of the abscess, which is essential for planning surgery (25). MRI is also the modality of choice for diagnosing compressive myelopathy. Leukocyte count, ESR, and CRP are usually elevated.
MRI depicts an intradural extramedullar collection, better defined on axial images, with intermediate signal on T1WI and most frequently hyperintense on T2WI. Postcontrast images show heterogeneous, diffuse subdural enhancement or a clear rim-enhancing fluid collection in the subdural situation. There may be cord impingement, edema, or malacia (22).
Surgical drainage together with systemic antibiotics is the treatment of choice (23,25). Without intervention, patients who are already with spinal compression signs would almost certainly not reverse the neurologic deficits. Because the rate of progression of neurologic impairment is difficult to predict and some patients became paralyzed within hours after the onset of neurologic deficit, laminectomy, evacuation of the puslike material, and debridement of infected tissues should be done as soon as possible (25,26).
Arachnoiditis
Arachnoiditis is an inflammatory condition of the spinal leptomeninges, manifested more commonly as subarachnoid adhesions involving the spinal roots, that may produce varying degrees of CSF blockage, subarachnoid cysts, syringomyelia, and, rarely, hydrocephalus. The most common form is lumbar spine adhesive arachnoiditis. Among its causes, most of them are iatrogenic; are agents injected into the subarachnoid space, like contrast media, anesthetic agents, and intradural steroids; infection, trauma, intradural, or extradural surgery; and intrathecal hemorrhage (Table 24.8). All contrast media used before the introduction of metrizamide and the later water-soluble nonionic myelographic agents are known to cause arachnoiditis, manifesting as intradural adhesions on subsequent myelography or MR in up to 60% to 70% of patients. Arachnoiditis has been cited as a cause of “failed back surgery” syndrome in up to 16% of patients. Patients with persisting symptoms after lumbar disc surgery often have a complex history of investigations and therapeutic procedures, many of which could be responsible for arachnoiditis. Therefore, multiple causative factors have been implicated, among them myelographic contrast media being used during the preoperative assessment, perioperative infection, therapeutic or inadvertent intraspinal injections of anti-inflammatory agents, and intrathecal hemorrhage or other forms of operative trauma.
The inflammatory reaction of arachnoiditis involves initially the influx of white blood cells in response to an insult to the subarachnoid space, foreign substance, or infectious agent. This is followed by infiltration of macrophages and mesenchymal cells, with the latter evolving into fibroblasts and producing collagen. In the more advanced phases, there is predominance of fibrinous exudates, and the cellular response is minimal. Usually the fibrinolytic process, which breaks down excessive scar tissue, limits this process. In arachnoiditis, it seems that there is a defect in the fibrinolytic pathway, and the fibrin-coated nerve roots and arachnoid membrane adhere to one another. These adhesions are subsequently reinforced by proliferating fibroblasts.
TABLE 24.8 Causes of Arachnoiditis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
When the arachnoiditis is local, the symptoms may be very specific and related to a particular lumbosacral root, and, most of the time, it is very difficult to determine whether they are related exclusively to arachnoiditis or to another associated condition like compression by a residual disc or osteophyte, or even the coexistence of epidural fibrosis.
The most common clinical signs of lumbar adhesive arachnoiditis are pain in the lower back and weakness and sensory loss in the lower limbs. Some patients refer to bladder and sexual dysfunction. An important observation, however, is that the clinical symptomatology of arachnoiditis may be complicated by multifactorial factors that include psychosomatic, legal, and employment-related aspects.
In a series of patients with benign lumbar arachnoiditis caused by previous myelography and/or surgery, acquired MR both before and after intravenous injection of gadopentetate dimeglumine correlated well with myelographic and postmyelographic CT, supporting the findings of other authors that arachnoiditis can be diagnosed with unenhanced MRI. Sagittal and mostly axial T2WI best demonstrate centrally clumped or peripheral adherent roots (Figs. 24.20 and 24.21), although these changes can also be seen on T1WI. In mild and severe cases of arachnoiditis, the diagnosis may be established if there are segmentally clumped roots centrally located or roots adherent to the wall in the lower lumbar thecal sac. Gadolinium chelate enhancement of the nerve roots is infrequent, inconspicuous, often uncertain, and not helpful in the diagnosis of arachnoiditis. In selected cases, however, the use of contrast media is indispensable (Fig. 24.22).
State-of-art MRI has replaced myelography as the reference examination to detect arachnoiditis. High-resolution FSE imaging permits reliable identification of the anterior and posterior roots for each spinal root within the thecal sac and multiple rootlets within each root sheet, further signs of arachnoiditis can be seen that should permit recognition of mild and even minimal cases (Table 24.9).
Syringomyelia and subarachnoid cysts are recognized complications of arachnoiditis, more commonly those associated to inflammation or infestation and tend to be more common in the thoracic region.
The main MR findings of syringomyelia associated to arachnoiditis are loss of the sharp cord–CSF interface resulting from obliteration of the subarachnoid space by arachnoid adhesions and septation of the syrinx on axial T1WI, probably representing
parallel areas of cavitation rather than within the same cavity. Arachnoid cysts, most of them located at the upper aspect of the syrinx, suggests a role in the development of cord cavitation (Fig. 24.23–24.25). Hydrocephalus may also rarely complicate arachnoiditis (Fig. 24.22).
parallel areas of cavitation rather than within the same cavity. Arachnoid cysts, most of them located at the upper aspect of the syrinx, suggests a role in the development of cord cavitation (Fig. 24.23–24.25). Hydrocephalus may also rarely complicate arachnoiditis (Fig. 24.22).
A rare manifestation of arachnoiditis is ossification of the leptomeninges, or arachnoiditis ossificans of the spine, that may result in severe neurologic decline. One has to consider that calcifications of the meninges are common. These calcifications are most often asymptomatic due to their relatively small size in relation to the spinal canal. This process of asymptomatic calcification is distinct from the pathologic one, which is due to chronic inflammation. Plain radiographs in only the most severe cases may identify it and CT and MRI are necessary complementary studies.
Acute Transverse Myelopathy
The term acute transverse myelopathy (ATM) refers to a monophasic focal inflammatory disorder of the spinal cord of unknown etiology that involves both halves of the spinal cord, producing paraplegia, a sensory impairment level, and sphincter dysfunction. Both terms ATM and acute transverse myelitis have often been used interchangeably in the literature, creating considerable confusion. ATM has inflammatory and noninflammatory causes such as demyelinating diseases, viral infections, postviral and postvaccinal processes, collagen vascular disorders, vascular disorders, paraneoplastic syndromes, and also may be idiopathic. There was a time when the term acute transverse myelitis was reserved for idiopathic cases, but currently the term ATM is also used to encompass the general clinical syndrome, whether or not the cause is known (27). In fact, a recent series showed that cases of ATM secondary to an identifiable cause are much more common than those still considered idiopathic (28). In addition, considering the fact that the clinical syndrome of ATM may have noninflammatory causes, such as vascular, traumatic, and compressive, the diagnosis of acute transverse myelitis is only possible after the exclusion of these conditions.
The Transverse Myelitis Consortium Working Group proposed the diagnostic criteria for idiopathic acute transverse myelitis (Table 24.10). The diagnosis of idiopathic ATM requires that all of the inclusion criteria and none of the exclusion criteria
are fulfilled. The diagnosis of disease-associated ATM requires that all the inclusion criteria are met and that the patient is identified as having an underlying condition listed among the disease-specific exclusions. Patients meeting all diagnostic criteria are considered to have definite idiopathic ATM, whereas those who do not meet the MRI or CSF criteria for inflammation have possible idiopathic ATM.
are fulfilled. The diagnosis of disease-associated ATM requires that all the inclusion criteria are met and that the patient is identified as having an underlying condition listed among the disease-specific exclusions. Patients meeting all diagnostic criteria are considered to have definite idiopathic ATM, whereas those who do not meet the MRI or CSF criteria for inflammation have possible idiopathic ATM.
TABLE 24.9 Magnetic Resonance Imaging Diagnosis of Arachnoiditis | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
TABLE 24.10 Criteria for the Diagnosis of Idiopathic Acute Transverse Myelitis | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||
ATM is characterized clinically by acute or subacute development of symptoms and signs of bilateral dysfunction in motor, sensitive, and autonomic nerves and nerve tracts of the spinal cord, frequently with a sensorial rostral level, which progresses to a nadir over 4 to 21 days from onset. It usually affects middle-aged adults, and the thoracic spine is most commonly involved, followed by the cervical spine.
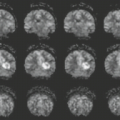
MRI may directly demonstrate a centrally located increased signal intensity on T2WI, usually occupying more than two-thirds of the cross-sectional area of the cord and extending more than three to four vertebral segments in length (Fig. 24.26). The spinal cord may be of normal caliber or slightly expanded, which in the latter case may even suggest a neoplasm. Cord expansion may be found in up to 47% of cases. After gadolinium administration, the abnormal areas may (Figs. 24.27 and 24.28) or may not show enhancement, and both patchy and diffuse patterns were described. It is important to note that MRI findings became one of the diagnostic criteria of ATM. The Transverse Myelitis Consortium Working Group proposed that at least one of the three following criteria is required for the diagnosis of ATM (1): MRI demonstration of abnormal gadolinium enhancement of the spinal cord, (2) CSF pleocytosis, or elevated CSF immunoglobulin G (IgG) index (3). They also proposed that if none of the inflammatory criteria are met at symptom onset, one should repeat MRI and lumbar puncture evaluation between 2 and 7 days, to determine whether these criteria are met. One drawback of these criteria is that cord enhancement is reported in only up to 38% to 53% of the cases (28).
There are two important considerations. First, in the emergency setting, a complete workup is not always done, and there is no certainty that all diagnosable conditions are excluded. Second, it remains to be determined how to distinguish, at disease onset, idiopathic ATM patients who do not have evidence of disseminated CNS disease from those with multiple sclerosis (MS) or neuromyelitis optica (NMO) (Table 24.11) (28). The evolution of idiopathic transverse myelitis is extremely variable, resulting in severe disabilities in about one-third of the patients (28). Follow-up MRI reflects this variability, showing resolution of the abnormal signal and return of the cord to a normal caliber when the evolution is favorable or to spinal cord atrophy in disabled patients (Figs. 24.29 and 24.30).
TABLE 24.11 Conditions that May Present with a Transverse Myelopathy (28) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree