and John P. Deveikis2
(1)
Department of Surgery, Division of Neurosurgery, and Departments of Radiology and Neurology, University of Alabama, Birmingham, AL, USA
(2)
Bayfront Medical Center, St. Petersburg, FL, USA
Abstract
Several classification schemes for spinal vascular lesions have been described (Borden et al., J Neurosurg 82: 166–179, 1995; Spetzler et al., J Neurosurg 96:145–156, 2002; Bao and Ling, Neurosurgery 40:75–81, 1997; Zozulya et al., Neurosurg Focus 20:E7, 2006). The following four-type system is the most commonly used, with several other spinal vascular lesions added for completeness: Type I: Dural arteriovenous fistula (dAVF); Type II: Intramedullary arteriovenous malformation (AVM); Type III: Juvenile AVM; Type IV: Intradural perimedullary AVF; Extradural arteriovenous fistulas; Spinal cord aneurysms; Intramedullary cavernous malformations; Vascular spinal tumours; Spinal cord ischaemic stroke. Technical aspects of endovascular treatment of spinal vascular lesions are discussed in Chap. 8, Extracranial embolization.
Several classification schemes for spinal vascular lesions have been described.1–4 The following four-type system is the most commonly used, with several other spinal vascular lesions added for completeness:
Type I: Dural arteriovenous fistula (dAVF)
Type II: Intramedullary arteriovenous malformation (AVM)
Type III: Juvenile AVM
Type IV: Intradural perimedullary AVF
Extradural arteriovenous fistulas
Spinal cord aneurysms
Intramedullary cavernous malformations
Vascular spinal tumours
Spinal cord ischaemic stroke
Technical aspects of endovascular treatment of spinal vascular lesions are discussed in Chap. 8, Extracranial Embolization.
20.1 Type I: Dural Arteriovenous Fistula
Type I lesions (aka angioma racemosum, angioma racemosum venosum, intradural dorsal AVF, long dorsal AVF, dorsal extramedullary AVF) consist of an abnormal communication between the radicular artery in the nerve root sleeve and the intradural venous system, causing venous hypertension (Fig. 20.1). They can be subclassified into type I-A and type I-B lesions, depending on whether there is one or more radicular feeding arteries.5,6
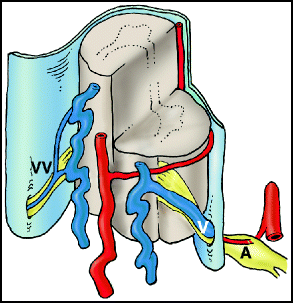

Fig. 20.1
Type I dural arteriovenous fistula (dAVF). There is a direct connection between a radicular artery (A) and a radicular vein (V) in the dura of the nerve root sleeve. Arterialization of the coronal venous plexus causes engorgement and congestion of the veins. Note that the contralateral radicular vein (VV) in this depiction is small; impairment of alternative routes of venous drainage is thought to contribute to the development of venous hypertensive myelopathy.
20.1.1 Epidemiology and Clinical Features
1.
Type I dAVFs are the most common spinal vascular lesion, representing approximately 70% of spinal vascular malformations.7
5.
6.
7.
Presentation:
8.
Imaging:
a.
MRI is the screening procedure of choice for spinal dAVFs.16 Spinal cord hyperintensity on T2-weighted images and postgadolinium enhancement on T1-weighted images are the most common findings.7,18 Cord signal changes usually extend for six or seven vertebral levels.7,17
b.
Catheter angiography is the gold standard for the workup of spinal dAVFs.16
i.
Selective injection of the thoracic and lumbar spinal arteries should be done first, since the majority of dAVFs are located in those regions.
ii.
When the artery of Adamkiewicz is found, imaging of the venous phase of the angiogram will fail to show normal filling of spinal cord veins in most cases of spinal dAVF.20 This is evidence of the severe venous hypertension in the cord.
iv.
Rarely, intracranial dural AVFs may drain inferiorly and mimic spinal dural AVFs clinically and on MRI.22–24
With intracranial fistulas draining to spinal cord veins, angiography of the artery of Adamkiewicz may show normal appearance of spinal cord veins in the venous phase.25 Thus, if a fistula is not found during spinal angiography, angiography of the cerebral vessels should be done.
v.
Dilated, tortuous veins draining a dAVF are characteristic findings, predominantly along the posterior surface of the spinal cord.
vi.
When a fistula is found, the adjacent levels should be imaged as well, because of the possibility of multiple radicular feeding arteries.
vii.
It has been reported that placement of a platinum coil in the major feeding artery can facilitate intraoperative fluoroscopic localization of the fistula.26
20.1.2 Pathophysiology
1.
Normal radicular veins have a constriction at the point where the vein passes through the dura, which prevents the transmission of arterial pressure into the valveless coronal venous plexus.28 Fistulas are usually located at this point or within the nerve root sleeve.29 The fistula is usually supplied by a meningoradicular branch of a segmental artery, although any artery supplying the dura may be involved.16 The intrathecal spinal venous system is valveless, and therefore arterial pressure is transmitted via the corresponding radicular vein into the perimedullary and spinal veins, causing venous hypertension, congestion and impairment of the spinal cord and nerve root microcirculation.30 Direct measurement of the coronal venous pressure during surgery found that the spinal cord venous pressure averages 74% of the simultaneous mean systemic venous pressure.30
2.
The aetiology of spinal dAVFs is not understood. Interestingly, in contrast to cranial dAVFs, in which venous sinus thrombosis is believed to contribute to the development of those lesions, prothrombotic conditions are not associated with spinal dAVFs.31
20.1.3 Management
The natural history of untreated spinal dAVFs is generally thought to be poor. An early series found that 50% of untreated patients became severely disabled (wheel-chair-bound) within 3 years of the onset of lower extremity weakness.37
Both surgery and endovascular treatment can be effective for treatment of type I lesions. Although surgery appears to be more curative, embolization is less invasive and some authors recommend an attempt at embolization prior to surgery. In a systematic review of 20 published clinical series, 98% of patients treated with surgery were reported to have successful obliteration of their fistulae, compared to only 46% with embolization.38 Complications were reported in 1.9% of surgical patients and 3.7% of embolization patients.
20.1.4 Surgical Considerations
1.
Neurophysiological monitoring with evoked potentials is not necessary, as manipulation of the spinal cord is not required.21
2.
A two-level hemilaminectomy is done to adequately expose the affected nerve root.
3.
The dura is opened in the midline and retracted laterally.
4.
The radicular draining vein is exposed where it penetrates the dura and is coagulated and divided.
Interruption of the radicular vein is an important step for successful cure of the fistula and usually leads to an immediate visible change in venous turgor, and the colour of the arterialized venous plexus may change from red to blue.
5.
If the fistula involves a thoracic nerve root, the root may be sacrificed to facilitate dural closure. Obviously, cervical and lumbar nerve roots should be preserved.
6.
In cases in which extradural drainage of the fistula is present, the entire fistula including portions of the draining vein should be excised and the intradural and extradural components should be divided to prevent recurrence.40
7.
Outcomes with surgery:
a.
A systematic review of surgical outcomes found that 55% of patients improved after surgery, 34% were stabilized, and 11% worsened.38 Only 33% of patients showed an improvement in micturition, and 11% worsened.
b.
A recent single center series of 154 surgical cases reported complete exclusion of fistula at first attempt in 95% and 96.6% of patients experienced improvement, and 6% worsened.41
20.1.5 Endovascular Considerations
1.
Case selection: Embolization should be done only when the anatomy of the lesion will permit obliteration of the nidus and proximal part of the vein.
Barriers to embolization include advanced atherosclerosis, arterial feeders too small to catheterize, and collateralization of the feeding vessel with normal spinal cord vessels.
b.
Embolization is most effective when the glue penetrates the proximal portion of the draining vein; if the glue does not reach the draining vein, the fistula may persist or recanalize. In an endovascular series, the fistula recurred in 68% of cases in which the glue did not reach the draining vein, compared with 50% of cases in which the glue did reach the draining vein.43
2.
Embolization is particularly useful in patients who are poor candidates for surgery, or in some cases as a temporizing measure, to reduce venous congestion until a definitive surgical procedure can be performed.44
3.
The embolization agent of choice is N-butyl cyanoacylate.
5.
6.
Reports on long-term outcomes after embolization are lacking. Clinical outcome data on embolization were insufficient for analysis in the systematic review discussed earlier.38
20.1.6 The Often Misunderstood Foix–Alajouanine Syndrome
In 1926, Foix and Alajouanine published a 42-page report of two cases of progressive myelopathy.32 An extensive pathological analysis was carried out, and the authors implicated vascular congestion, as reflected by spinal cord vessel thickening, in the pathological process afflicting both patients. In the decades since this report, numerous authors have included spinal cord venous thrombosis as a central feature of the Foix–Alajouanine Syndrome.6,44,50–53 Indeed, both authors of this handbook were taught, during their training, that Foix–Alajouanine Syndrome is equivalent to progressive, malignant spinal cord venous thrombosis. In the actual report, however, Foix and Alajouanine emphasized that in their two cases no thrombosis was present.54 They described vessel wall thickening, without luminal narrowing or obliteration of cord vessels, and excluded the presence of vascular malformations within the cord. The inclusion of thrombosis as a feature of Foix–Alajouanine Syndrome is a myth that has been perpetuated most likely because the original report was written in French. In retrospect, it seems likely that both patients in the original report had progressive myelopathy due to type I dural AVFs,44 an entity that had not yet been recognized at the time of the publication.54
20.2 Type II: Intramedullary Arteriovenous Malformation
Type II lesions (aka glomus or classic AVM) consist of an AVM within the substance of the spinal cord. The nidus can be classified as compact or diffuse, and they often have multiple feeding vessels arising from the anterior and posterolateral spinal arteries (Fig. 20.2).
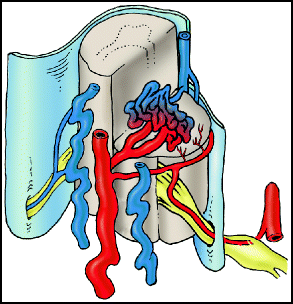

Fig. 20.2
Type II intramedullary AVM. Intramedullary arteriovenous malformation with a compact nidus is illustrated.
20.2.1 Epidemiology and Clinical Features
1.
4.
6.
Spinal cord AVMs are located in the cervical cord in 30% of cases and in the thoracolumbar cord in 70%, which is proportional to the volume of the spinal cord at each segment.39
7.
8.
Presentation:
a.
Symptoms may be acute or progressive, although in most cases the symptoms develop relatively rapidly.62
b.
d.
Haemorrhage at presentation may be more common among children with cord AVMs compared with adults.65
e.
Re-haemorrhage appears to happen at higher rates for spinal cord AVMs compared with brain AVMs, occurring in 10% of patients at 1 month and in 40% within the first year after the initial haemorrhage.64
g.
Conus AVMs may present with myelopathy or radiculopathy.
Supply of conus AVMs can be provided by the artery of Desproges-Goteron (aka the cone artery). This artery may arise from the internal iliac artery or its branches.67
9.
Imaging:
a.
MRI is highly sensitive and is able to detect all, or nearly all spinal cord AVMs.51,68,69
i.
MRI findings include a focal dilatation of the cord around the lesion, an area of low signal around the nidus on T1- and T2-weighted imaging that corresponds to haemosiderin deposition, and multiple flow voids (on axial images) and serpentine structures (on sagittal and coronal images) due to feeding and draining vessels.
iii.
Subacute haemorrhage appears as increased signal on FLAIR and T1-weighted images.
b.
Catheter angiography remains the gold standard for the evaluation of spinal cord AVMs.39 A complete angiogram to characterize all feeding and draining vessels, look for aneurysms, and distinguish the lesion from associated normal vessels is necessary to plan treatment.
i.
Selective injection of numerous arteries is necessary to fully characterize a cord AVM, as feeding vessels may arise from sources as far afield as the occipital, ascending pharyngeal, vertebral, ascending and deep cervical, supreme intercostal, intercostal, lumbar, and the lateral and median sacral arteries.64
20.2.2 Management
The natural history of untreated intramedullary AVMs is not clear. Progressive evolution of symptoms, by either worsening myelopathy or subsequent haemorrhages, is reported in 31–71% of patients observed over several years.12,65,70,71 Because spinal cord AVM anatomy is variable and the risk of potential complications on any procedure involving the cord is relatively high, decision making about the management of these patients is highly individualized. Patients with a cord AVM consisting of a compact, surgically accessible nidus may be good candidates for surgery. Embolization may be a useful adjunct to surgery, or, in some cases, may provide symptomatic relief without necessarily obliterating the lesion. There is a school of thought that holds that partial embolization of spinal cord AVMs, even with impermanent materials (such as PVA) may provide an (impermanent) improvement in symptoms such as pain and myelopathy.70 The notion that partial treatment of cord AVMs lowers the risk of haemorrhage, which is generally believed not to be the case with intracranial AVMs, is more controversial.
20.2.3 Surgical Considerations
1.
2.
With appropriate case selection (i.e., by operating on patients with a relatively compact, surgically accessible nidus), angiographic obliteration of the lesion can be achieved in up to 94% of cases.62
Surgery for diffuse spinal cord AVMs has been reported. In a series of three cases, the lesion was obliterated in all; neurological outcome improved in one patient and deteriorated slightly to mildly in the other two patients.75
3.
Surgical approach is via a standard laminotomy. Exposure should extend at least one level above and one level below the lesion. A small myelotomy is done in the posterior median sulcus, and the spinal cord is split between the two posterior columns. Alternatively, a posterolateral myelotomy, done in the dorsal root entry zone between two or more nerve roots, can provide access to lateral lesions.
4.
In one series, delayed imaging (mean follow-up, 8.5 years) in patients with no evidence of residual AVM on early postoperative imaging detected new draining veins in 23% of cases.62
5.
Outcomes with surgery:
a.
As expected, surgical results are better with compact AVMs compared with AVMs with a diffuse nidus.4
b.
20.2.4 Endovascular Considerations
2.
3.
Biondi et al. advocate routine yearly spinal angiography and embolization with PVA, regardless of symptoms.70 Despite frequent lesion revascularization, 63% of patients demonstrated long-term clinical improvement with this strategy. Worsening of symptoms after embolization was observed in 20% of patients.
4.
5.
Choice of embolic agent:
a.
The first-line agent for embolization of any cord AVM should be NBCA or Onyx, provided that the microcatheter tip can be placed within the nidus.
b.
If the microcatheter tip can be placed close to the nidus, but beyond angiographically visible normal spinal cord vessels, NBCA is still a good choice.
c.
Particulate embolization should be reserved for cases in which the microcatheter tip is relatively proximal to the lesion. Flow-directed embolization with a particulate agent will theoretically carry most of the particles past normal branches and into the nidus. Injection should be done slowly and carefully, without attempting to completely occlude the nidus.
i.
Sizing of the particulate agent is based on the following reasoning: Because the normal anterior spinal artery diameter is 340–1,100 μm and the normal sulcal artery diameter is 60–72 μm, particles with a diameter of 150–250 μm should pass through the anterior spinal artery and into the AVM nidus without entering the normal sulcal arteries.77,78
ii.
Although PVA is the particulate agent of choice by some operators, PVA size is highly variable. The authors of this handbook prefer to use 100–300 μm Bead BlockTM Microspheres (Terumo Medical Corporation, Somerset, NJ) or 100–300 μm Embosphere® Microspheres (BioSphere Medical, Inc., Rockland, MA).
20.2.5 Radiosurgery
In a preliminary report of stereotactic radiosurgery for intramedullary AVMs, six of seven patients at least 3 years from treatment had a significant reduction in AVM volume, and one patient with a conus lesion was found to have complete angiographic obliteration.79
20.3 Type III: Juvenile Arteriovenous Malformation
Type III lesions (aka juvenile, metameric, or extradural-intradural AVM) are complex AVMs that have both intradural and extradural components, and typically involve the spinal cord, vertebra, and paraspinal muscles (Fig. 20.3). The portion of the nidus involving the spinal cord typically has neural tissue within its interstices. They are high-flow lesions, and may have a bruit over the lesion. They are extremely rare and may appear as part of Cobb syndrome (see below). Patients are typically children or young adults (thus the term juvenile), and present with pain and/or myelopathy. These AVMs typically have multiple feeding vessels arising from diverse locations, such as the vertebral arteries, radicular arteries, and other cervical vessels. Catheter angiography of a juvenile AVM can be somewhat like shining a small flashlight on an elephant in a dark room – only a part of the lesion can be seen with injection of contrast into any particular artery. These lesions are extremely difficult to treat. Although complete lesion resection by staged embolization followed by surgery has been reported,80,81 fatal complications with this approach have also been reported.6
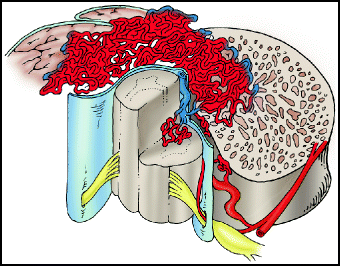

Fig. 20.3
Type III juvenile AVM. Type III lesions characteristically extend across tissue planes from the spinal cord into the adjacent bone and muscle.
20.3.1 Cobb Syndrome
Cobb syndrome is a rare congenital disorder with a slight male predominance characterized by a combination of vascular skin naevi and a spinal vascular lesion occurring within the same metameres (i.e., the skin lesions are found in the dermatomes corresponding to the spinal levels where the spine lesion is). It was first described by Stanley Cobb, a resident of Harvey Cushing, in a report of a 8-year-old boy who presented with paraplegia.82 The child had naevi over the 9th to 12th ribs, and at surgery he was found to have an angioma of the thoracic spinal cord. The spinal vascular lesion that occurs as part of Cobb syndrome may be a type III AVM, or a less complex lesion such as a perimedullary AV fistula.83 Therefore, type III spinal vascular lesions and Cobb syndrome are not exactly synonymous.
20.4 Type IV: Intradural Perimedullary Arteriovenous Fistula
Type IV lesions (aka perimedullary, or ventral intradural AV fistulas) are located on the pial surface of the spinal cord, usually on the anterior or lateral surface (Fig. 20.4). They consist of a fistula between a spinal cord artery or arteries and the coronal venous plexus, and there is often a varix at the artery-to-vein transition site. Type IV lesions account for 13–17% of all spinal vascular lesions.12,55 They were originally subdivided into type I, II, or III lesions depending on size and complexity;84 subsequent authors have adopted a type A–C system.2 (Table 20.1). An association with split cord malformation has been reported.85 Type B and C lesions are associated with Rendu-Osler-Weber and Cobb syndrome.86
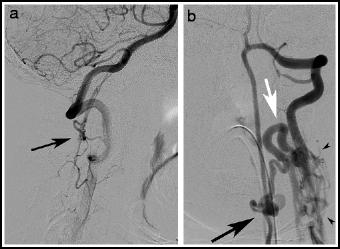

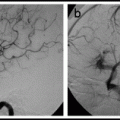
Fig. 20.4
Type IV perimedullary AV fistulas. Lateral view, vertebral artery injection (a), showing a small cervical Type A ventral perimedullary AV fistula (arrow) fed by several small pedicles arising from the vertebral artery. Frontal view, vertebral artery injection (b), showing a large Type C ventral perimedullary AV fistula (black arrow), fed by two large arteries, one from superior to the fistula and the other coming from an inferior source. Drainage is into a single vein (white arrow), which empties into the spinal venous plexus (arrow heads).
Table 20.1
Subtypes of perimedullary AV fistulas
Type | Arterial supply | Venous drainage | Primary mode of treatment |
|---|---|---|---|
A | Anterior spinal artery only | Ascending | Surgery |
B | Anterior and posterolateral spinal arteries | Ascending | Embolization ± surgery |
C | Anterior and posterolateral spinal arteries | Dilated segmental veins | Embolization |
1.
Type A: The least common type IV lesion. Small, single-vessel fistula supplied by the anterior spinal artery, usually located on the anterior surface of the conus or on the upper part of the filum terminale. Venous drainage is slow and is in a rostral direction.
2.
Type B: Larger, multiple-vessel fistulae supplied by the anterior and posterolateral spinal arteries, usually located on the posterolateral or anterolateral surface of the conus. Venous drainage is slow and is in a rostral direction.
3.
Type C: The most common type IV lesion (Fig. 20.4). These lesions consist of a single giant fistula supplied by multiple, enlarged feeders from the anterior and posterolateral spinal arteries. They are located on the thoracic cord, or, less commonly the cervical cord.55,87 Venous drainage is rapid and into segmental veins.
20.4.1 Clinical Features
2.
No sex predominance.
3.
The location of these lesions along the spinal axis is bimodal, with most occurring at the thoracolumbar junction, particularly at the conus, and, to a lesser extent, in the upper cervical region.88
5.
6.
Imaging:
a.
b.
Apparent diffusion coefficient (ADC) values may be reduced, indicating vasogenic oedema, and may normalize after treatment.91
d.
Catheter angiography is necessary to make a clear diagnosis of a perimedullary fistula and plan therapy.
20.4.2 Management
Because type IV lesions are rare, published series are limited, and firm conclusions about treatment cannot be made. Estimations of the natural history of untreated lesions suggest progression from myelopathy to paraplegia within 5–7 years, and a high incidence of repeated haemorrhage in patients presenting with haemorrhage.12,55,84,87,88 Most authors recommend prompt diagnosis and treatment of perimedullary fistulae to minimize neurological injury.12,55,84,87 The objective of treatment should be occlusion of the fistula. The absence of a nidus facilitates surgery;55 most lesions can be effectively obliterated with either surgery or embolization, or a combination of both.3,55,86,93,94
1.
2.
3.
20.4.3 Extradural Arteriovenous Fistula
Spinal extradural AV fistulas (aka epidural AV fistulas, extradural AVMs, or perivertebral AVMs) are rare.98–103
1.
Classification. The following classification system was recently proposed:104
a.
Type A
Extradural AV fistula with both an arterialized epidural venous plexus and an arterialized vein that pierces the dura, travels intradurally, and drains into the venous plexus that surrounds the spinal cord. This lesion differs from a Type I dural AV fistula in that in a Type A extradural AV fistula the intradural draining vein arises from a extradural venous pouch, whereas in a Type I dural fistula the AV shunt lies within the nerve root sheath and drains directly into an intradural drain.2,102,104
b.
Type B1
Extradural AV fistula that drains only into the Batson plexus with compression of the thecal sac and with myelopathy.
c.
Type B2
Extradural AV fistula that drains only into the Batson plexus without compression of the thecal sac and without myelopathy.
2.
Imaging
Type A lesions typically are associated with cord oedema and perimedullary flow voids on MRI.104 Spinal angiography is necessary for diagnosis.105 DynaCT (injection of 40 mL of 20% diluted iodinated contrast during 20 s of rotation) provides complementary information to catheter angiography and can help in clarifying the relationships between the fistula and the spinal canal, spinal cord, nerve roots, and surrounding structures.104
20.4.4 Spinal Cord Aneurysms
Spinal cord aneurysms are rare. Most are discovered as flow-related lesions associated with an intramedullary AVM, although isolated spinal cord aneurysms do occur.106–108 and can cause subarachnoid haemorrhage.108 Spinal aneurysms may explain SAH when no intracranial aneurysm is found; often the patient complains of back or neck pain in addition to and often beginning before the classic headache of SAH. Particularly large aneurysms may present with symptoms of myelopathy or radiculopathy.106 There appears to be a strong association with developmental vascular anomalies, as metameric angiomatosis was found in 43% of patients in a series of cord aneurysms.59 Spinal aneurysms are typically fusiform in shape and thus very difficult to treat directly with endovascular techniques. Surgery of spinal cord aneurysms with trapping106 and wrapping109 has been reported. Disappearance or a decrease in size of the aneurysm was observed in several patients who underwent treatment of an associated intramedullary AVM.58 Importantly, even solitary spinal cord aneurysms may spontaneously regress without treatment.108
20.4.5 Intramedullary Cavernous Malformation
Cavernous malformations (aka cavernomas) are distributed along the entire neuroaxis and represent 5–12% of all spinal vascular lesions.110,111 See Chap. 16 for a discussion of intracranial cavernous malformations. Rarely, spinal cavernous malformations may be found in an epidural location.112 Spinal cavernomas are histologically identical to those in the brain. They are angiographically occult and have a characteristic appearance on MRI.111 As many as 40% of patients with a spinal cavernoma have similar intracranial lesions.113 As in the brain, spinal cord cavernomas are frequently associated with a developmental venous anomaly.109 A systematic review found a female predominance with a peak age at presentation in the forth decade.114 Symptoms is highly variable and the natural history of symptomatic spinal cavernomas is far from clear. Annual rates of symptomatic rehaemorrhage range from 0%115 to 66%.116 Although surgical series report relatively good results,111,114,117 expectant management of selected patients may also be reasonable.115 A recent report of long-term results in 80 patients after resection found:118
1.
Immediately after surgery:
a.
11% of patients were worse
b.
83% were the same
c.
6% improved
2.
Perioperative complications occurred in 6% of patients.
3.
At mean follow-up of 5 years:
a.
10% of patients were worse
b.
68% were the same
c.
23% were improved
4.
Long-term complications, including kyphotic deformity, stenosis and spinal cord tethering, occurred in 14% of patients.
20.4.6 Vascular Spinal Tumours
Vascular spinal tumors include haemangioma, haemangioblastoma, metastatic tumours, aneurysmal bone cyst, osteoblastoma, angiosarcoma, haemangiopericytoma, angiofibroma, angiolipoma, haemangioendothelioma.
Indications and technique for endovascular embolization of these lesions is discussed in Chap. 8.
20.4.7 Spinal Cord Infarction
Any part of the central nervous system may suffer ischaemic injury, and this may occur in the spinal cord. Spinal cord ischaemia in the cord is uncommon and may be misdiagnosed as other pathological processes such as transverse myelitis. Cord ischaemia is becoming increasingly recognized due increasing clinical awareness of this condition, especially in association with stent-grafting for aortic aneurysms. Brain ischaemia is discussed in Chap. 17.
20.4.7.1 Epidemiology and Clinical Features
1.




Spinal cord ischaemia is uncommon, representing 1.2% of stroke cases.119
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree