Fig. 9.1
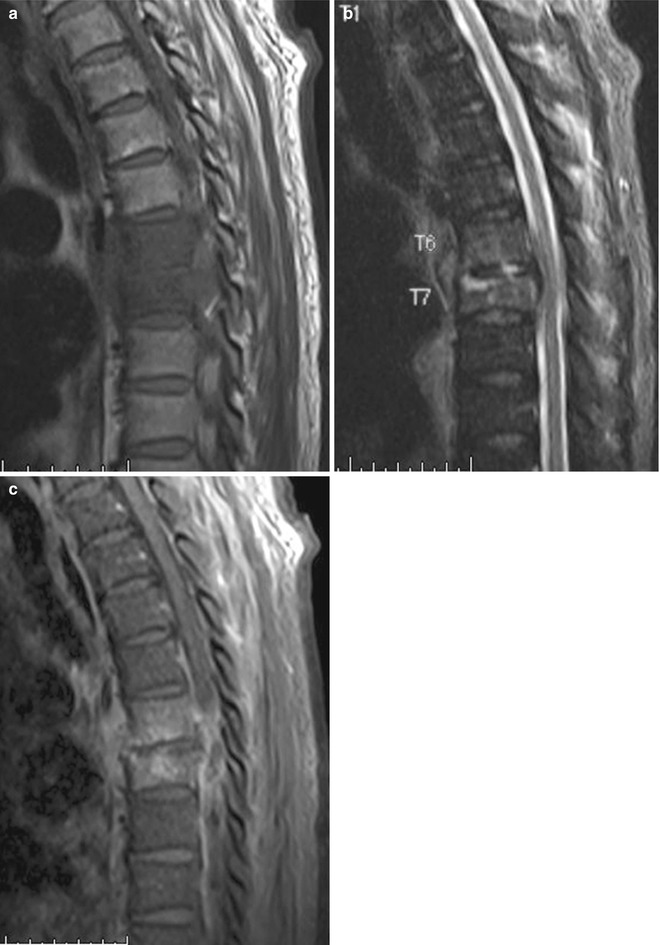
Lumbosacral transitional vertebra. Sagittal (a) T1-W and (b) fat-suppressed T2-W MR images show lumbarization of the first sacral segment. (c) Without the whole spine localizer, accurate determination of lumbosacral transitional vertebra would not have been possible
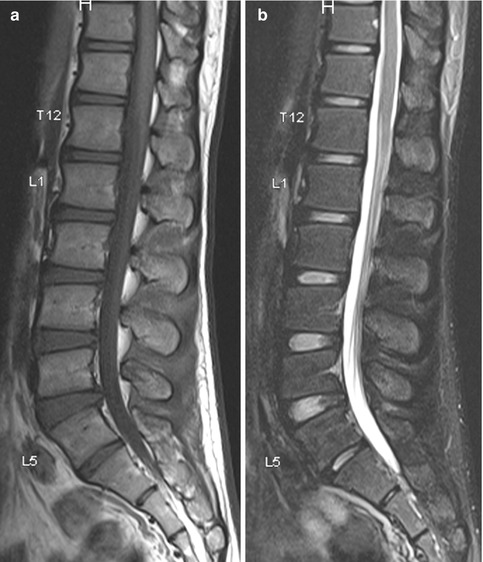
Fig. 9.2
Lumbosacral transitional vertebra. Sagittal (a) T1-W and (b) fat-suppressed T2-W MR images show a sacralized L5 vertebra. Labeling was done by counting caudally using a whole spine localizer
The clinical significance of the identification of transitional vertebra is twofold. Firstly, there is a purported association with low back pain in the sacralization of the L5 segment, and secondly, there is the possibility of error in the numbering of the vertebra, resulting in surgery or intervention performed at the wrong level. The association of low back pain with transitional lumbar anatomy is controversial and has been termed Bertolotti syndrome, after Bertolotti (1917) who first described it. It is currently thought to be due to various etiologies, such as pathology at the level above the transition, arthropathy at the unilateral anomalous articulation or contralateral facet joint, as well as extra-foraminal stenosis secondary to the presence of a broadened transverse process. Various mechanical factors as a result of abnormal anatomy are thought to be the common root cause of these entities (Konin and Walz 2010).
The incidence of wrong-level spine surgery has been estimated at 12.8 occurrences per 10,000 lumbar diskectomies and 7.6 per 10,000 cervical diskectomies (Jhawar et al. 2007). Another survey of 415 surgeons revealed an estimated prevalence of 1 in 3,110 procedures for wrong-level spine surgery, with the majority involving the lumbar spine (Mody et al. 2008). Variant spinal anatomy with erroneous numbering of the vertebral segments is often the cause of wrong-level spine surgery. It is crucial for the surgeon to correlate intraoperative radiographs with preoperative MR images for the confirmation of disk levels before proceeding. It is also important for the reporting radiologist to alert the surgeon of the presence of transitional anatomy. This is to avoid confusion regarding the numbering of the vertebral segments as well as to identify the correct site for intervention. In MRI, localizers of the whole spine are invaluable to avoid this pitfall when imaging the lumbar spine, as it allows for the numbering of the vertebrae starting from the cervico-cranial junction (Peh et al. 1999; Thawait et al. 2012) (Fig. 9.1).
9.2.2 Fracture Mimics
Fractures of the spine are common in the setting of trauma. Radiography is often the initial modality for imaging the spine in minor trauma. However, the use of CT as a primary modality for the evaluation of spinal trauma is becoming increasingly common, especially in major trauma where other abdominal and thoracic injuries are present. The advent of multi-detector CT allows for high-resolution sagittal and coronal reformats of the spine, which makes for accurate and detailed evaluation of spinal trauma. MRI has a complementary role in the evaluation of the spinal canal, the spinal cord, as well as the paravertebral soft tissues, including the supportive ligaments of the spine. There are several pitfalls in the interpretation of these imaging modalities, which may result in false-positive results. These include limbus vertebra, prominent Schmorl nodes, degenerative anterior wedging, as well as normal variants in the atlas and axis, all of which may mimic fractures.
9.2.2.1 Limbus Vertebra and Schmorl Node
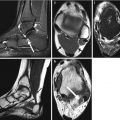
Limbus vertebra was first described by Schmorl in 1927 (Schmorl and Junghanns 1971) and results from the herniation of the nucleus pulposus into the body of the adjacent vertebra near its anterior margin, causing separation of a triangular bony fragment (Fig. 9.3). This has been well demonstrated on diskographic procedures, where intradiscal contrast is seen to extend between the fragments of a limbus vertebra. It is postulated to arise from mechanical stress factors during flexion, similar to a Schmorl node (Fig. 9.4), which occurs in the central portion of the endplate rather than marginally (Hellstadius 1949). It is most commonly seen in the anterosuperior corner of a single vertebral body in the mid-lumbar spine. Posterior herniations are rare but can be the cause of lumbosacral radiculopathy and cauda equina compression, especially in adolescents and young adults (Goldman et al. 1990). The radiographic appearance of a limbus vertebra may mimic a fracture, infection, or tumor, particularly in children, where it can appear as an irregular and apparently destructive process along the anterior margin of the vertebra. In adults, the identification of a limbus vertebra or Schmorl node is less difficult as they commonly display sclerotic and well-defined margins, in contrast to the appearance of diskitis or acute fracture. The ability to recognize this condition is important to avoid unnecessary further investigations or biopsy (Ghelman and Freiberger 1976).
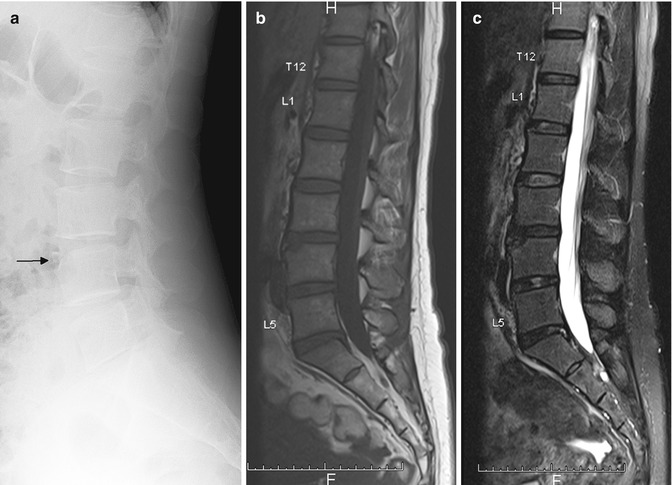
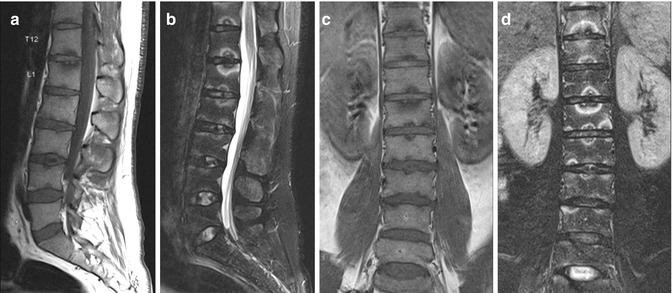

Fig. 9.3
Limbus vertebra mimicking fracture. (a) Lateral radiograph shows a triangular bony fragment arising from the anterosuperior corner of L4 vertebral body (arrow). Sagittal (b) T1-W and (c) fat-suppressed T2-W MR images show herniation of the nucleus pulposus into the body of the adjacent vertebra near its anterior margin, causing separation of a triangular bony fragment

Fig. 9.4
Schmorl nodes mimicking fractures. Sagittal (a) T1-W and (b) fat-suppressed T2-W and coronal (c) T1-W and (d) fat-suppressed T2-W MR images show multiple Schmorl nodes, with demonstration of the typical concentric ring pattern of T1-hypointense and T2-hyperintense signals around the herniated disks at the vertebral endplates
9.2.2.2 The Atlas and Axis
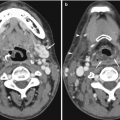
The first two cervical vertebrae have unique anatomical morphologies compared to the remaining vertebrae. Developmental anomalies can result in variant anatomy, which are often mistaken for fractures in the setting of spinal trauma. It is thus important for the radiologist to be familiar with these anomalies. The most commonly seen anomaly in the atlas is a posterior cleft or rachischisis, being found in 4 % of adult autopsy specimens. This can be seen on radiographs and usually confirmed on CT as a well-corticated linear defect in the midline of the posterior arch. Rarely, there is coexistence of an anterior cleft, resulting in a “split atlas” (Smoker 1994) (Fig. 9.5).
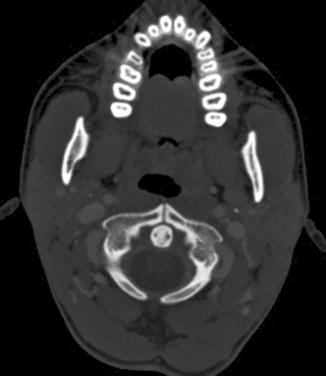

Fig. 9.5
Split atlas mimicking fracture. Axial cervical spine CT image shows a corticated linear defect in the anterior and the posterior arches of the atlas, in keeping with a “split atlas”
Developmental anomalies of the axis involve the odontoid process in the vast majority of cases and may similarly be mistaken for fractures. A persistent ossiculum terminale or Bergmann ossicle results from the failure of fusion between the terminal ossicle and the rest of the odontoid process, which normally takes place by the age of 12 years. It appears as a small triangular or pyramidal ossicle just above the odontoid process and can thus mimic a type I odontoid peg fracture. Differentiation is normally made on CT, where well-corticated margins of the ossicle are demonstrated. An os odontoideum is an independent osseous structure seen in place of the normal odontoid peg above the body of the axis (Fig. 9.6). This can simulate a type 2 odontoid peg fracture. The differentiating features lie in the outline of the upper border of the body of the axis, as well as the anterior arch of the atlas. Os odontoideum is usually associated with a well-corticated convex upper border of the body of the axis, as well as a hypertrophied anterior arch of C1, which appears rounded on lateral or sagittal views rather than its usual semilunar shape. A type 2 odontoid peg fracture, on the other hand, shows a flat, non-corticated upper border of the body of the axis and a normal anterior arch of C1 (Smoker 1994) (Fig. 9.7).
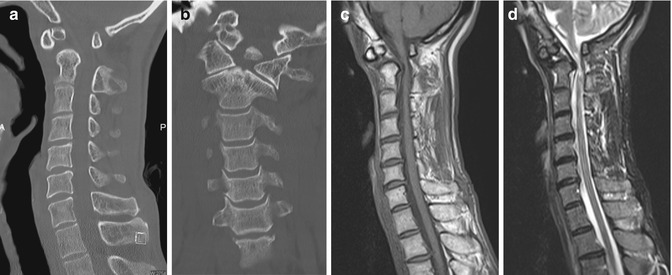
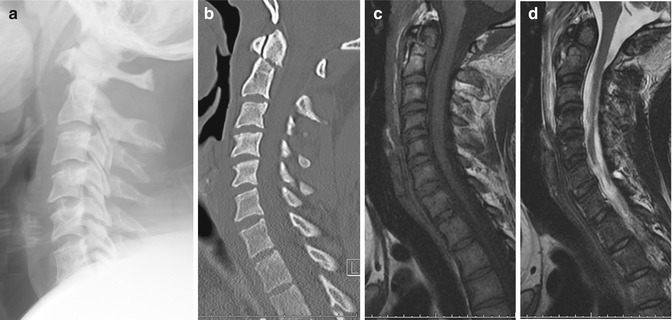

Fig. 9.6
Os odontoideum mimicking fracture. (a) Sagittal and (b) coronal cervical spine CT images show a separate odontoid fragment with well-corticated margins. Sagittal (c) T1-W and (d) fat-suppressed T2-W MR images show normal marrow signal within the os odontoideum

Fig. 9.7
Type 2 odontoid peg fracture. (a) Cervical spine radiograph shows widening of the prevertebral soft tissue. (b) Reconstructed sagittal cervical spine CT image confirms a slightly displaced odontoid process fracture. Sagittal (c) T1-W and (d) fat-suppressed T2-W MR images show extensive prevertebral hematoma in addition to the odontoid fracture
9.2.3 Spinal Cord
Filum terminale lipoma and ventriculus terminalis are benign intraspinal conditions that can be considered normal variants. Their typical appearance and features are described.
9.2.3.1 Filum Terminale Lipoma
Filum terminale lipoma or fatty filum terminale (Fig. 9.8) is a normal variant seen as a thin linear strip of fatty tissue in the dorsal half of the thecal sac, extending for a varying length below the conus medullaris to the sacrum, in an asymptomatic patient. It demonstrates absence of contrast enhancement as well as low signal on fat-suppressed images. It is differentiated from a tethered cord by the normal position of the conus medullaris and the absence of thickening of the filum terminale. In a symptomatic patient, intraspinal lipoma needs to be considered. This is characterized by a larger lipomatous mass, with an associated thickening of the filum terminale of >2 mm (Ross et al. 2004). Postmortem studies have reported a 4–6 % incidence of occult fibrolipomas of the filum terminale in what were thought to be otherwise normal spinal cords (Brown et al. 1994). Epidural lipomatosis is a separate entity commonly associated with obesity and chronic steroid use. Diffuse proliferation of fatty tissue within the dural sac can cause neural compression, producing neurologic symptoms as well as back pain (Tehranzadeh et al. 2000).
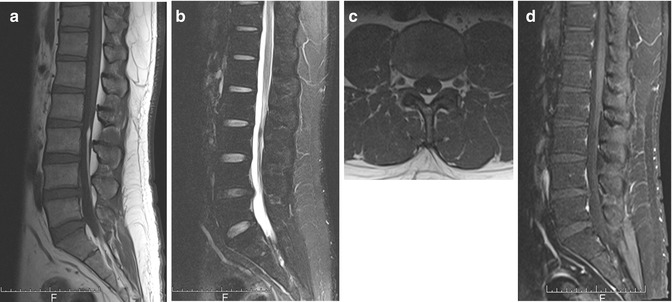

Fig. 9.8
Filum terminale lipoma. Sagittal (a) T1-W, (b) fat-suppressed T2-W, and (c) axial T1-W MR images show a thin linear strip of fatty tissue in the dorsal half of the thecal sac. (d) There is signal dropout and absence of contrast enhancement as well as signal dropout on contrast-enhanced fat-suppressed T1-W MR image. These features are typical of filum terminale lipoma
9.2.3.2 Ventriculus Terminalis
Ventriculus terminalis or terminal ventricle is the cystic dilatation of the distal central spinal cord canal, sometimes referred to as the fifth ventricle. It is located within the conus medullaris with cerebrospinal fluid (CSF) signal intensity on MRI, typically 2–4 mm in diameter and rarely exceeding 2 cm in length. It is often an incidental finding and is rarely the cause of symptoms. It is important to distinguish this from cystic neoplasm or hydrosyringomyelia on imaging. The presence of calcification, septation, nodule, enhancement, eccentric location, or signal changes in the adjacent spinal cord would be considered suspicious for a cystic neoplasm. Hydrosyringomyelia is only restricted to the distal cord in 2.5 % of cases and usually extends for a longer length compared to the terminal ventricle. A terminal ventricle may be indistinguishable from a small localized syrinx; however, the subsequent management for either remains close monitoring of neurologic symptoms and interval follow-up imaging. One must be wary of truncation or phase ghosting artifacts which can also mimic the appearance of a dilated terminal ventricle or syrinx (Coleman et al. 1995; Ross et al. 2004; Ciappetta et al. 2008).
9.3 Spinal Lesions
Many lesions in the spine can have similar appearances or overlapping features which may trip up the unwary radiologist. Misinterpretation may result in the wrong treatment prescribed and a delay in diagnosis. Specific imaging signs assist in distinguishing between these lesions and enable the radiologist to favor one diagnosis over another. Close correlation with clinical signs and symptoms, as well as biochemical markers, is stressed. However, in some circumstances, the clinical and imaging signs may be equivocal, and biopsy is required for definitive diagnosis.
9.3.1 Osteoporotic Versus Pathological Fracture
Compression fractures of the vertebral bodies are seen with increasing incidence in the elderly population, with osteopenia or osteoporosis being the most common cause. The spine is also a common site for metastatic disease in the same age group, accounting for up to 39 % of all bone metastases and which may result in pathological fractures (Yuh et al. 1989). Chronic, nonneoplastic compression fractures are easily recognized on MRI by their lack of abnormal marrow signal. However, an acute or subacute fracture may have imaging features similar to a malignant compression fracture, making differentiation between the two difficult. This is of great clinical significance as it may impact on the staging, treatment plan, and prognosis of a patient with a known primary malignancy (Jung et al. 2003).
There are several useful imaging signs described in the literature that can assist the radiologist in the differentiation of osteoporotic from malignant fractures. In osteoporotic fractures, there is usually preservation of some normal fatty marrow signal within the vertebral body (Fig. 9.9). Most vertebral metastases do not result in compression fractures until the entire body is infiltrated by tumor, causing structural weakening from bone destruction (Yuh et al. 1989). The involvement of the pedicles or posterior elements is also a useful sign. Benign compression fractures rarely extend into the pedicle or posterior elements. Fluid or gas within the vertebral body can be seen as vacuum clefts on CT or conventional radiographs and is usually a sign of a benign fracture as it implies the presence of a cavity that is not filled with neoplastic tissue (Baur et al. 2002). The ability to discern a discrete fracture line of low T1 and T2 signal intensity within the vertebral body has also been reported to be a feature of osteoporotic fracture. The presence of an associated soft tissue component, including epidural mass and particularly a focal paraspinal mass, is suggestive of a neoplastic process (Fig. 9.10). Involvement of the cervical or upper thoracic (T1–T5) spine is more often seen in metastatic fractures. A convex posterior border of the fractured vertebra is somewhat helpful; however, it can also be seen in up to 20 % of osteoporotic fractures (Jung et al. 2003; Griffith and Guglielmi 2010).
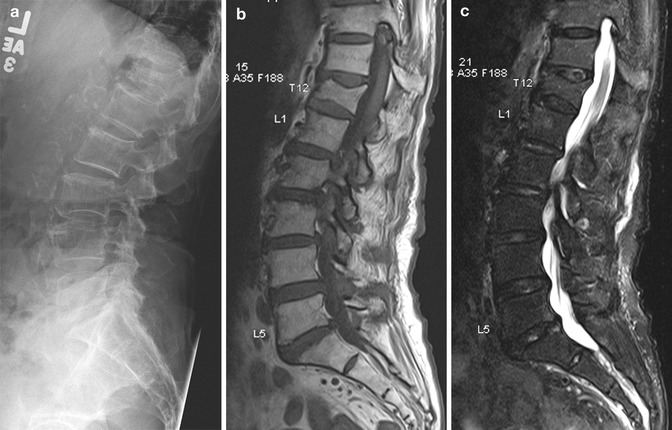
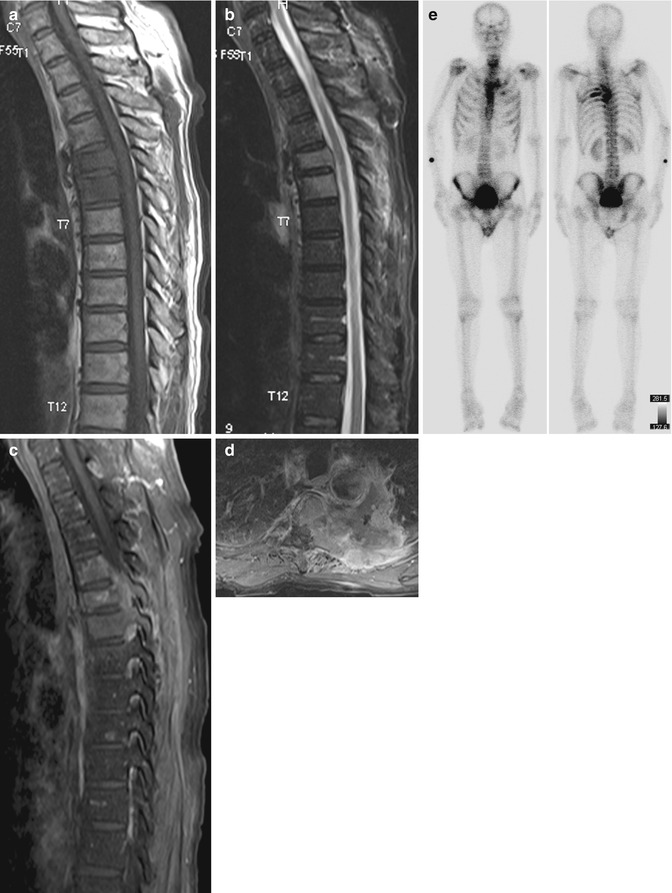

Fig. 9.9
Osteoporotic compression fracture. (a) Lateral radiograph of the lumbar spine shows collapse of the T12 vertebral body with degenerative changes and osteopenia. Sagittal (b) T1-W and (c) fat-suppressed T2-W MR images show preservation of normal fatty marrow signal within the vertebral body, in keeping with an osteoporotic fracture

Fig. 9.10
Pathological fracture due to metastases. Sagittal (a) T1-W, (b) fat-suppressed T2-W, and (c) sagittal and (d) axial contrast-enhanced fat-suppressed T1-W MR images show a T5 vertebral body compression fracture. There is T1-hypointense and T2-hyperintense replacement of the normal fatty marrow signal with involvement of the pedicles and the posterior elements, with lesion enhancement and an associated large soft tissue mass. Although not compressed, the adjacent T4 and T6 vertebrae are also similarly involved. (e) Tc-99m diphosphonate bone scintiscans show multiple bone metastases from left lung primary cancer
The presence of a known primary malignancy is not a useful parameter, as up to one-third of vertebral fractures in this group of patients are due to osteoporosis and not metastases (Fornasier and Czitrom 1978). Administration of intravenous gadolinium contrast agent is also of limited use as both acute osteoporotic and neoplastic fractures can show enhancement. Several studies have demonstrated the value of using diffusion-weighted imaging (DWI) in differentiating benign from pathological fractures of the vertebra (Raya et al. 2006; Karchevsky et al. 2008). Neoplastic fractures tend to show increased DWI signal due to higher cellularity and reduced extracellular fluid component. However, this appearance may be confounded by the presence of “T2 shine-through” effect. The use of the apparent diffusion coefficient (ADC) as a quantitative measure of diffusivity of water molecules has been proposed to address this. Several studies have found a cutoff value of more than 1.0 × 10−3 mm2/s as a predictor of osteoporotic fracture (Chan et al. 2002; Balliu et al. 2009; Tang et al. 2007). The described features are summarized in Table 9.1. The application of a combination of these signs and imaging techniques aids the radiologist in more accurate discrimination between benign and pathological fractures of the spine. However, a biopsy may still be required for definitive diagnosis in equivocal cases.
Table 9.1
Differentiating osteoporotic from malignant fractures
Osteoporoticfracture | Malignantfracture | |
|---|---|---|
Marrow | Some normalsignal preserved | Near completereplacement |
Posteriorelements | Not usuallyinvolved | Commonlyinvolved |
Vacuum cleft | May be present | Absent |
Low signalfracture line | Present | Absent |
Soft tissuecomponent | None or minor | Prominent, focal |
Convex posteriorborder | Up to 20 % of cases | Common |
DWI signal(ADC value) | >1.0 × 10−3 mm2/s | <1.0 × 10−3 mm2/s |
9.3.2 Postoperative Spine
Image interpretation of a postsurgical spine is complex and challenging. Many factors have to be taken into consideration, including the type of surgery performed, time since the procedure, implants used, preoperative imaging findings, as well as the nature of the current symptoms (Van Goethem et al. 2002). The presence of metallic implants further complicates matters when imaging with CT or MRI, producing streak or susceptibility artifacts which obscure vital structures and may even render the study uninterpretable or nondiagnostic. Several techniques have been employed to reduce the beam-hardening artifacts from metallic prostheses on CT. These include the use of high peak voltage and tube current, as well as narrow collimation and thin sections during image acquisition. The use of thick sections with lower kernel values during reformatting can also reduce streaking (Thakkar et al. 2012). The early days of MRI of the instrumented spine were plagued by severe artifacts, limiting its use. This has improved in recent times with the use of modern pulse sequences, as well as with the introduction of titanium implants which resulted in reduced artifacts (Viano et al. 2008). When imaging with MRI, the following parameters may help to attenuate the effect of metallic prostheses:
Fast spin-echo sequences
Inversion recovery fat suppression
Swapping phase- and frequency-encoding directions
View-angle tilting
Increasing readout bandwidth
Decreasing voxel size (Thakkar et al. 2012)
9.3.2.1 Recurrent Disk Protrusion Versus Epidural Scar
Diskectomy is one of the most common types of spine surgery. It involves the removal of herniated disk material to decompress underlying neural elements. It is approached via various degrees of resection of the posterior elements, as listed below:
Laminotomy: resection of part of the lamina
Laminectomy: resection of the whole width of the lamina
Laminectomy with facetectomy: laminectomy with resection of part of the facet joint (Thakkar et al. 2012)
Normal and expected appearances following diskectomy include high T2 signal intensity within the intervertebral disk up to 2 months following surgery, as well as disk enhancement up to 6 weeks postsurgery. Changes in the endplates simulating Modic type 1 appearance of low T1 and high T2 signal intensities may also be encountered. Soft tissue in the epidural space adjacent to an irregular annular margin may appear similar to preoperative disk protrusion, which will subsequently involute into epidural scar tissue. Nerve root enhancement can be seen in the immediate postsurgical period, reducing in intensity at 3 months and becoming absent by 6 months. Any enhancement seen following this period should be considered pathological. Defects in the lamina reflect the amount of bone that has been resected. Surrounding disruption and edema in the paraspinal muscles are present with an enhancing subcutaneous tract. These changes are seen in almost all patients up to 3 weeks after surgery and gradually resolve after 3 months. Small fluid collections, which are usually sterile, can be a normal finding (Babar and Saifuddin 2002).
Postoperative complications encountered following diskectomy include recurrent disk protrusion, epidural scar formation, diskitis, arachnoiditis, and postsurgical collections. One of the more common indications for postoperative imaging of the spine is the development of recurrent symptoms, usually 6 months following the procedure. A recurrent disk protrusion or epidural scar formation can both produce similar clinical symptoms. The incidence of recurrent disk protrusion and symptomatic epidural scar is 5–11 % and 8–14 %, respectively. It is important to be able to differentiate between them as a recurrent disk protrusion may warrant repeat surgery, which is contraindicated in fibrosis. Gadolinium-enhanced MRI is the investigation of choice for evaluating these lesions.
A recurrent disk protrusion is usually polypoidal, has smooth margins, and demonstrates low signal intensity on both T1- and T2-weighted images. It is contiguous with the underlying disk, unless it is sequestered, in which case it may mimic an intraspinal mass or a neurogenic tumor (Fig. 9.11). On the other hand, scar tissue is usually irregular in outline and shows slightly higher intermediate signal intensity. Although both can cause mass effect, the presence of thecal sac retraction is more suggestive of a scar. The early enhancement pattern further helps to separate the two lesions. A recurrent disk protrusion, being avascular, shows either no enhancement or peripheral enhancement due to surrounding fibrotic or granulation tissue (Fig. 9.12). In contrast, scar tissue shows heterogeneous enhancement (Fig. 9.13). It is important to perform the scan soon after contrast administration as peripheral enhancement of a recurrent disk protrusion can spread from the outside in and mimic scar tissue. These imaging characteristics are summarized in Table 9.2. The findings described should be taken in the clinical context as these features may also be found in asymptomatic patients (Babar and Saifuddin 2002; Thakkar et al. 2012).
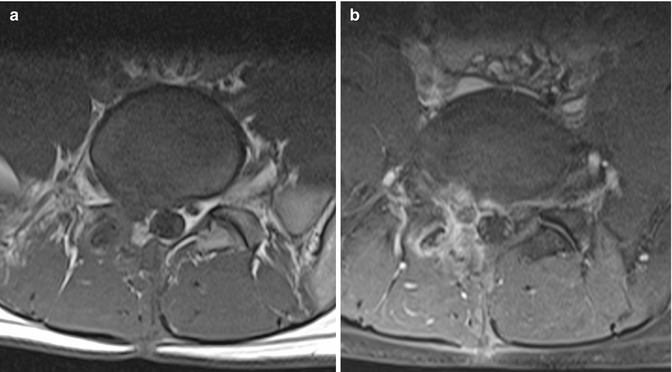
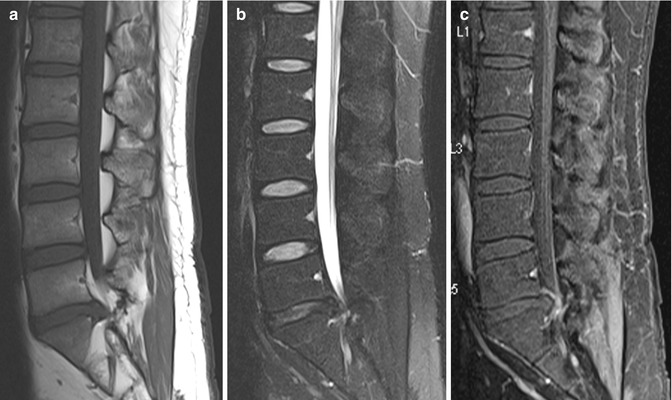
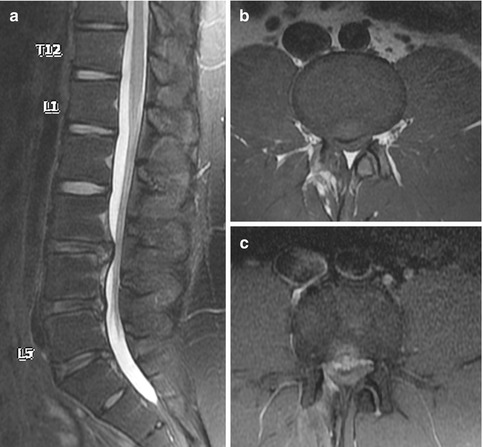

Fig. 9.11
Sequestrated disk mimicking a neurogenic tumor in a patient presenting with recurrent backache following previous L5/S1 hemilaminectomy and diskectomy. Axial (a) T1-W and (b) contrast-enhanced fat-suppressed T1-W MR images show a focal hypointense mass at the right lateral recess. It is indistinguishable from the adjacent nerve root on the unenhanced image. There is intense inflammatory rim enhancement surrounding the mass which is clearly separated from the adjacent compressed nerve root, consistent with an avascular sequestrated disk fragment

Fig. 9.12
Recurrent disk protrusion post-diskectomy in a patient who had a previous L5/S1 left hemilaminectomy and diskectomy. Sagittal (a) T1-W, (b) fat-suppressed T2-W, and (c) contrast-enhanced fat-suppressed T1-W MR images show focal L5/S1 disk protrusion causing thecal sac indentation. The recurrent disk protrusion is hypointense on all sequences, with thin rim enhancement due to the surrounding granulation tissue

Fig. 9.13
Epidural scar post-diskectomy in a patient who had a previous L3/4 microdiskectomy. (a) Sagittal fat-suppressed T2-W, (b) axial T1-W, and (c) contrast-enhanced fat-suppressed T1-W MR images show a T1- and T2-hypointense but moderately enhancing lesion indenting the anterior thecal sac at L3/4 level, consistent with an epidural scar at the site of diskectomy
Table 9.2
Differentiating recurrent disk protrusion from epidural scar
Recurrent diskprotrusion | Epidural scar | |
|---|---|---|
Contour | Polypoidaland smooth | Irregular |
Mass effect | Present | Present |
Thecal retraction | Absent | May be present |
MRI signal | Low on T1and T2 | Intermediate |
Enhancement (early) | None orperipheral | Central,heterogeneous |
9.3.2.2 Postoperative Collections
Small postsurgical sterile seromas can be seen in the immediate postoperative period and usually decrease in size on follow-up. Pseudomeningoceles may form following spinal surgery due to a tear in the dura. This is due to herniation of the dural sac with CSF contents into the paraspinal region, appearing as a thin-walled, well-marginated fluid collection posterior to the thecal sac. Uncomplicated lesions follow CSF fluid signal characteristics on all sequences (Fig. 9.14). Abscesses, on the other hand, typically have thick enhancing walls and show higher T1 and lower T2 signal intensities due to proteinaceous content, as well as marked enhancing inflammatory changes in the surrounding tissues. However, complicated pseudomeningoceles with hemorrhage or proteinaceous contents may demonstrate complex signal intensities and peripheral contrast enhancement, simulating an abscess (Ross 2000). Clinical correlation is important for the assessment of postsurgical fluid collections, and aspiration may be necessary for final diagnosis if there is a high clinical suspicion of an infected collection.
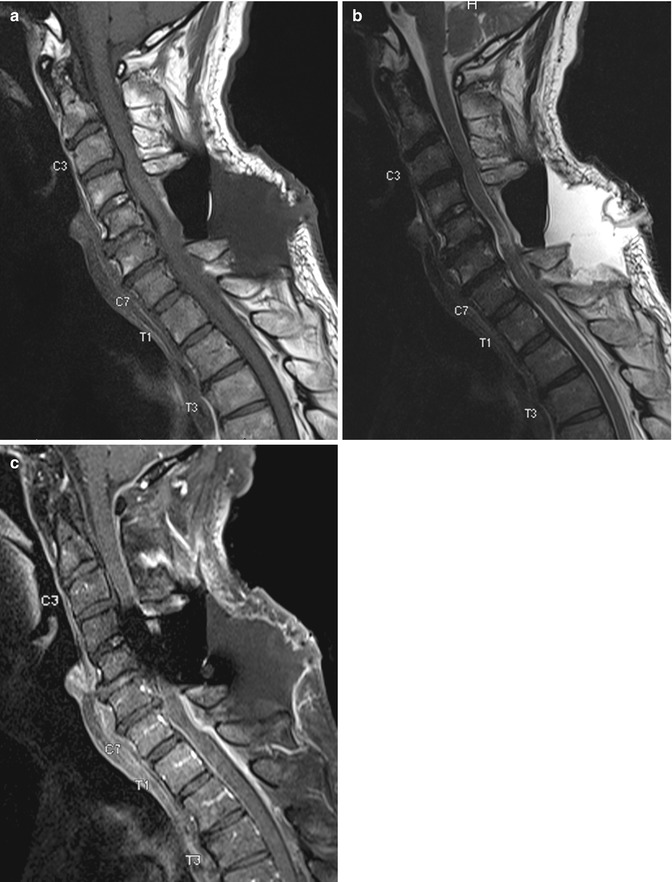

Fig. 9.14
Postoperative fluid collection. Sagittal (a) T1-W, (b) fat-suppressed T2-W, and (c) contrast-enhanced fat-suppressed T1-W MR images show a large postoperative fluid collection with air-fluid level located posterior to C4–T1 vertebral level. There is associated thin rim enhancement
9.3.3 Infection and Mimics
Spinal infections are an important aspect of spine imaging and account for 2–4 % of all skeletal infections (Maiuri et al. 1997). They may arise from distant septic foci via hematogenous spread or by direct extension from infections in adjacent tissues. Urinary tract infections are a well-known source of infection. Direct inoculation can also occur as a result of trauma or following spinal surgery or procedures. Staphylococcus aureus is the most common organism isolated, accounting for at least a third of all cases (Mylona et al. 2009; Yoon et al. 2010). The lumbar spine is most often affected (50 %), followed by the thoracic spine (35 %), with the remaining 15 % occurring in the cervical spine (Van Tassel 1994). MRI is the modality of choice for the assessment of suspected infectious spondylitis due to its high sensitivity and specificity (Tins and Cassar-Pullicino 2004). However, spondylitis may show atypical features, which make accurate diagnosis difficult. Furthermore, degenerative and noninfectious inflammatory conditions may have similar imaging features and can mimic spondylitis. Tuberculous spondylitis occasionally demonstrates distinctive features which enable differentiation from pyogenic spondylitis (Hong et al. 2009).
9.3.3.1 Pyogenic Versus Tuberculous Spondylitis
Pyogenic spondylitis has an insidious clinical course with nonspecific symptoms such as focal back pain, fever, malaise, and weight loss. Patients typically present late, up to several months from the onset of symptoms. Radiographs, although insensitive in the early stages of the condition, are often positive due to late presentation. Findings of endplate destruction, loss of disk space, and paraspinal soft tissues are seen in up to 89 % of cases (Mylona et al. 2009). MRI has proven to be the most useful modality in the imaging of spinal infections. Classical MRI features of pyogenic spondylitis include fluid signal intensity in a single disk space with low T1 signal in the adjacent endplates and associated loss of cortical definition (Fig. 9.15). Following intravenous administration of gadolinium contrast agent, the disk and adjacent vertebral marrow may show varying patterns of enhancement. Paravertebral and epidural extension in the form of inflammatory phlegmon or abscess can occur in more advanced disease, with similar inflammatory signal patterns on T1- and T2-weighted images and diffuse or rim enhancement. On follow-up, MRI features may not correlate with clinical progress and may even show worsening findings despite clinical improvement (Gillams et al. 1996). Therefore, imaging findings cannot be taken in isolation in the follow-up of patients with spondylitis. The patient’s clinical symptoms and laboratory markers should be assessed in conjunction with imaging features.