3
Stomach
Gastric Neoplasms
Overview
 Adenocarcinoma (>90%), gastric lymphoma (∼3% to 5%), gastrointestinal stromal tumors (GISTs) (∼3%)
Adenocarcinoma (>90%), gastric lymphoma (∼3% to 5%), gastrointestinal stromal tumors (GISTs) (∼3%)
Clinical Presentation
 Weight loss, early satiety, abdominal pain, nausea, vomiting
Weight loss, early satiety, abdominal pain, nausea, vomiting
 Dysphagia if tumor is in the proximal stomach (cardia)
Dysphagia if tumor is in the proximal stomach (cardia)
 Gastric adenocarcinoma metastasis
Gastric adenocarcinoma metastasis
• Virchow’s node: Metastasis to the left supraclavicular node
• Sister Mary Joseph nodule: Metastasis to the periumbilical region
• Krukenberg tumor: Metastasis to the ovary
• Blumer’s shelf: Metastasis to the pouch of Douglas
Diagnosis
 EGD is the gold standard for tissue diagnosis
EGD is the gold standard for tissue diagnosis
 EUS to assess for depth of invasion and lymphadenopathy
EUS to assess for depth of invasion and lymphadenopathy
 CT abdomen/pelvis and CXR for staging purposes
CT abdomen/pelvis and CXR for staging purposes
Treatment
 Adenocarcinoma
Adenocarcinoma
• Diagnostic laparoscopy to assess for metastatic disease
• Surgical resection with 5 cm margins with D1 or D2 nodal dissection
• Neoadjuvant or adjuvant chemotherapy depending on the stage
 Lymphoma
Lymphoma
• All are nonHodgkin type, most are low grade MALT (mucosal associated lymph tissue)
• Low-grade MALT: Likely a result of chronic Helicobacter pylori infection
 Antibiotic treatment for H. pylori
Antibiotic treatment for H. pylori
 Radiation ± chemotherapy for persistent disease after H. pylori treatment
Radiation ± chemotherapy for persistent disease after H. pylori treatment
• High-grade MALT: Chemotherapy and radiation therapy
 GIST
GIST
• Arises from interstitial cells of Cajal (intestinal pacemaker cells)
• Due to c-kit mutation
• Resection with negative margins
• Consider imatinib (Gleevec) if
 tumor >5 cm in size
tumor >5 cm in size
 more than 5 mitotic figures per 50 high-power field
more than 5 mitotic figures per 50 high-power field
 nongastric location
nongastric location
 tumor rupture
tumor rupture
 KIT—positive unresectable, metastatic, or recurrent disease
KIT—positive unresectable, metastatic, or recurrent disease
RADIOLOGY
Gastric Cancer
 Plain film findings
Plain film findings
• Gastric mass is usually not seen on plain radiographs
• Omental calcified metastases may sometimes be visible
 Upper GI findings
Upper GI findings
• If small, gastric adenocarcinomas will manifest as a raised ulcer with surrounding mucosal edema
 Folds are often thickened, irregular, or nodular
Folds are often thickened, irregular, or nodular
 Ulcerations, if present, are irregular in shape and do not extend beyond the gastric lumen
Ulcerations, if present, are irregular in shape and do not extend beyond the gastric lumen
 If the antrum is involved, it may be severely narrowed or obstructed
If the antrum is involved, it may be severely narrowed or obstructed
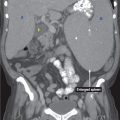
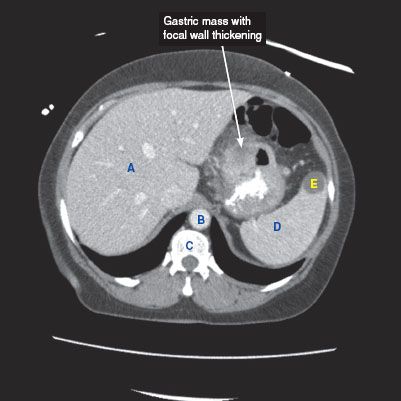
 CT findings (Fig. 3.1)
CT findings (Fig. 3.1)
• Focal wall thickening with or without ulceration, mass, or diffuse wall thickening
• CT is superior to barium studies to evaluate for the extent of disease
• Extension of tumor into adjacent organs and omental carcinomatosis can be seen
• Presence or absence of regional lymphadenopathy (adenopathy in the left gastric, porta hepatis, and peripancreatic regions) and presence of liver metastases can be evaluated
FIGURE 3.1
A. Liver
B. Descending aorta
C. Vertebra
D. Spleen
E. Splenic cyst
Gastric Lymphoma
 Upper GI findings
Upper GI findings
• Focal or diffuse gastric fold thickening
• Mass with nodular margins and luminal narrowing may be seen
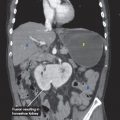
 CT findings (Fig. 3.2)
CT findings (Fig. 3.2)
• Focal or diffuse fold thickening, which can be associated with regional lymphadenopathy
FIGURE 3.2 A–C
A. Liver
B. Kidney
C. IVC
D. Descending aorta
E. Vertebra
F. Spleen
G. Small bowel loops
H. Bladder
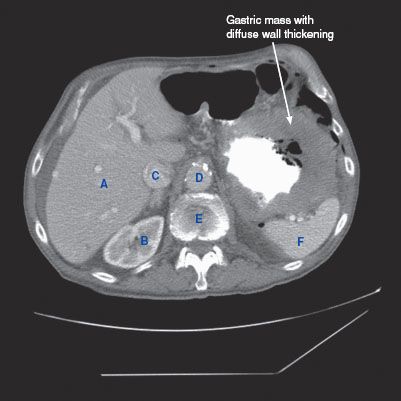
FIGURE 3.2 A

FIGURE 3.2 B

FIGURE 3.2 C
GIST Tumor
 Plain film findings
Plain film findings
• Nonspecific mass indenting or displacing the gastric bubble may be seen
• Upper GI Findings
 Intraluminal filling defect arising from the wall, forming smooth, obtuse angles with the rest of the stomach
Intraluminal filling defect arising from the wall, forming smooth, obtuse angles with the rest of the stomach
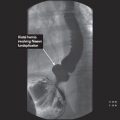
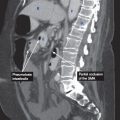
 CT findings (Fig. 3.3)
CT findings (Fig. 3.3)
• Mass arising from the gastric wall, usually with an exophytic growth pattern
• Central areas of low attenuation indicate hemorrhage or necrosis
 MRI findings
MRI findings
• Solid portions of tumor are T1 hypointense (pre-contrast) and T2 hyperintense
• Hemorrhage within tumor will manifest with variable T1 and T2 signals
• Heterogeneous enhancement
FIGURE 3.3 A–C
A. Liver
B. Descending aorta
C. Vertebra
D. Spleen
E. IVC
F. Kidney
G. Psoas muscle
H. Adnexal cyst
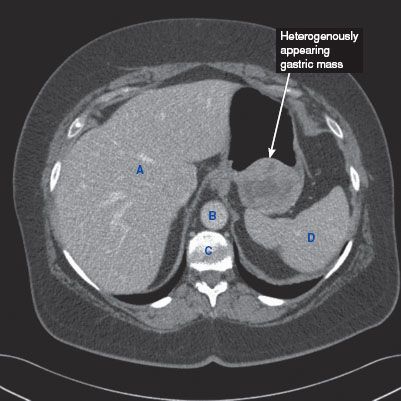
FIGURE 3.3 A
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree