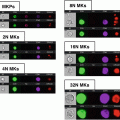
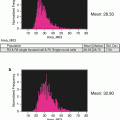
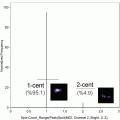
Fig. 1
Schematic representation of the analysis using an example of data acquired after staining for L-PHA and TCR. (a) Sequence of analysis steps. (1) A histogram for gradient RMS in bright-field images (Channel 01) can be used to gate for cells in focus. (2) A scatter plot of aspect ratio/area in Channel 1 (bright-field) is used to gate for single cells, within focused events. (3) The intensity of α/β TCR and L-PHA staining is used to gate on double positive cells within focused single cells. (b) Graphic representation of parameters analyzed from the double positive cells selected. (1) The level of co-localization at the membrane was quantified as the bright detail similarity. (2) Histogram represents the intensity of L-PHA staining on the membrane of α/β TCR+ L-PHA+ cells. (3) Panel showing examples of single cell bright-field (Ch01) and merged (TCR in red and L-PHA in green) images, showing co-localization between TCR α/β and L-PHA
4 Notes
1.
If you do not have any information about using your probe with the available imaging flow cytometer, you should start by titrating it. Perform the protocol described herein, but with different concentrations of probe. The ideal concentration of probe(s) allow you to visualize cells with a good, but not saturated signal (see Note 8 ), using the same settings for all probes.
2.
Ideally, cells should be counted and divided equally for each condition. But when having a limited number of cells, cell suspension can be divided in the 4 Eppendorfs, as following: 500 μl—double staining TCR-PE/L-PHA; 166 μl—single staining TCR-PE; 166 μl—single staining L-PHA- FITC; 166 μl—negative control (isotype + Streptavidin-FITC).
3.
Samples single staining TCR-PE (tube c) should be resuspended in 60 μl of PBS 1× and kept on ice until FIXATION.
4.
Fixation is a critical step that should be optimized to determine if it is better to fix before or after staining with the specific probes. Furthermore, it must be tested what is the best for fixation (paraformaldehyde, formaldehyde, methanol), since it may influence cells morphology and protein distribution, affecting the quality of images and consequently evaluation of the parameters of interest.
5.
Samples are resuspended in 60 μl of PBS 1× because the ImageStreamX will run samples with a minimum of 50 μl. The number of cells per sample advised by the manufacturer is approximately 1 × 106 cells (up to 5 × 106 cells) in a final volume of 50 μl, in a 1.5 ml microcentrifuge tube, but we found that samples with a lower number of cells can still be analyzed, although acquisition will take a long time.
6.




Ideally acquisition in the ImageStream cytometer should be performed immediately after staining. As this is not always practical, fixation allows keeping the cells in cold PBS 1× for a couple of days. Imaging should however be performed as soon as possible.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree