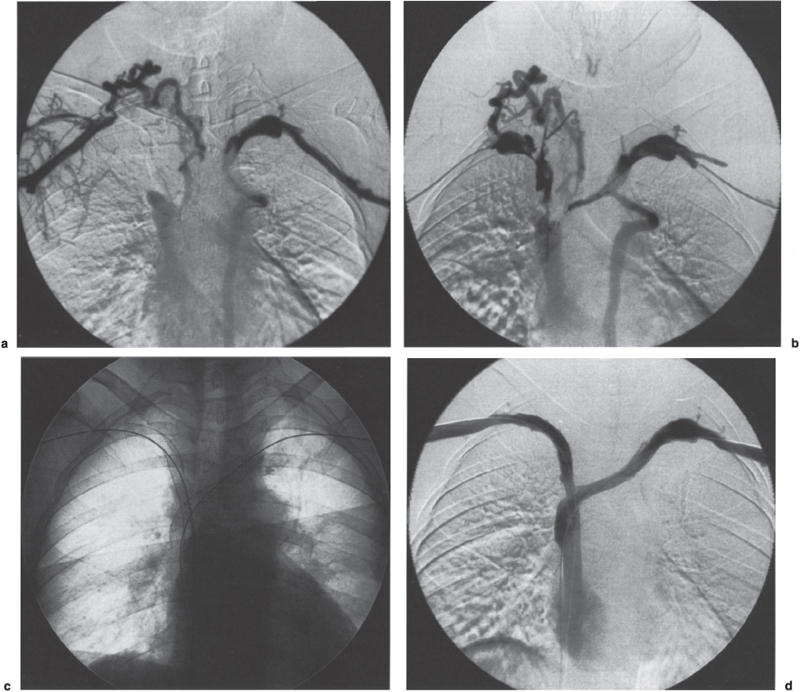
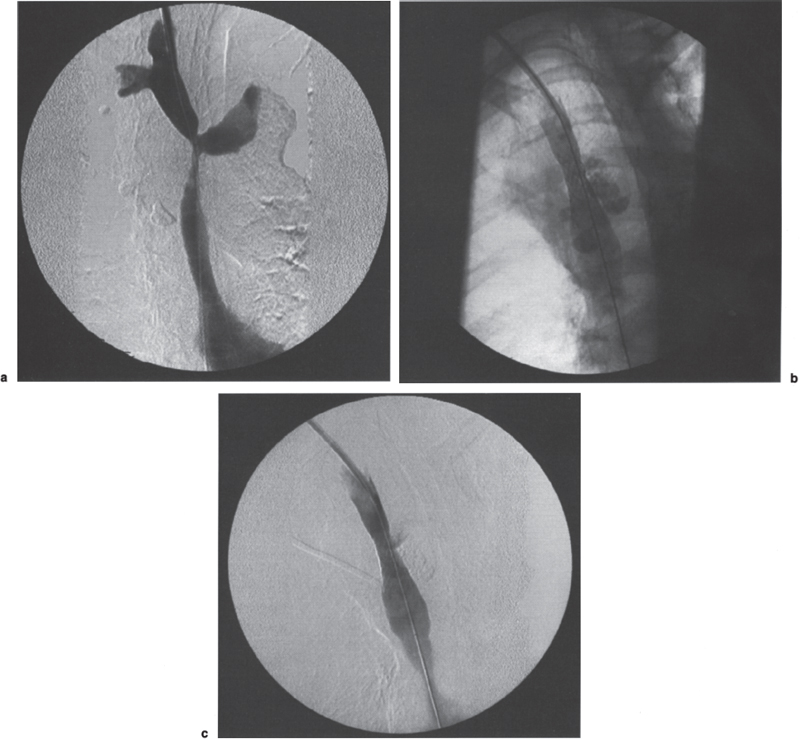
Superior Central Venous Stenosis and Occlusion The superior central venous system includes the superior vena cava and the axillary, subclavian, jugular, and innominate veins. Management of stenosis or occlusion of these veins presents a significant challenge to physicians. The etiology of superior central venous stenoses and occlusions includes a wide spectrum of processes, such as malignancy, central venous catheterization, high flow related to hemodialysis access (fistula or graft), extrinsic anatomic impingement, congenital and acquired webs, fibrosing mediastinitis, and deep vein thrombosis from a variety of other causes. The clinical presentation of patients with superior central venous stenosis or occlusion may be viewed as a continuous spectrum. At one end of the spectrum, the patient may be asymptomatic and the abnormality detected incidentally; at the other end of the spectrum, the patient is extremely symptomatic and the stenosis or occlusion is potentially life threatening, as in superior vena cava (SVC) syndrome. Regardless of the etiology of the central vein stenosis or occlusion, interventional radiologic techniques have played a significant role in the treatment of these lesions. This role has developed through a combination of technologic advances as well as relatively poor results with many of the surgical and nonsurgical alternatives. As yet, however, we are unable to offer definitive therapy in most cases, despite many advances in the treatment of central vein stenoses and occlusions. The treatment of superior central venous stenoses and occlusions will depend on the symptoms, cause, and location and, in cases of occlusion, duration. For the purposes of discussion, superior central venous stenoses and occlusions are divided into two broad categories: malignant and benign. This discussion is followed by description of the interventional radiological techniques used to treat venous thrombosis, stenosis, and nonthrombotic (chronic) occlusion: thrombolytic therapy, atherectomy, angioplasty, and metallic stents. Patients typically present with clinical symptoms directly related to venous encasement and subsequent thrombosis. Nowhere is this process more dramatic than when it involves the SVC, commonly referred to as SVC syndrome. Superior Vena Cava Syndrome First described in 1757 by Hunter, SVC syndrome is caused by partial or complete obstruction of the SVC as a result of a variety of malignant and benign entities (Table 29–1).1 The signs and symptoms of SVC syndrome include face, neck, and arm swelling; shortness of breath; stridor; headaches and other central nervous system (CNS) symptoms; cough; dysphagia; and syncope.2 Physical findings may include visible dilated tortuous collateral veins in the face, neck, and chest; papilledema; facial cyanosis; and pleural effusion.3–6 Also, life-threatening complications, such as laryngeal or cerebral edema, may occur.7 In extreme cases, coma, seizures, and death ensue. Malignant disease accounts for 85 to 90% of the cases of SVC syndrome.4–6,8–10 The most common malignant causes are bronchogenic carcinoma, lymphoma, and metastatic disease.11,12 SVC syndrome caused by malignancy is usually fatal within 6 to 7 months without treatment. Overall survival and symptom resolution depend on the ability to treat the underlying disease. Tumors Lung cancer (primary or metastatic) Lymphoma Thymoma Thyroid adenoma Neuroblastoma Plasmacytoma Liposarcoma Fibrosing (sclerosing) mediastinitis (tuberculosis, sarcoidosis, histoplasmosis) Substernal goiter Ascending aortic aneurysm (luetic aneurysms, atherosclerotic, dissecting aneurysms) Bronchogenic cysts Intravascular foreign bodies (pacemaker wires, catheters) Postsurgical (repair of tetralogy of Fallot, LeVeen shunts, mustard procedure, ventriculoatrial shunts) Radiation therapy Constrictive pericarditis Release of pericardial tamponade Heart or heart–lung transplant surgery Treatment of SVC syndrome must be individually tailored and targeted to the underlying etiology.3 Furthermore, the treatment must work expeditiously to provide relief of the severe or life-threatening symptoms. Immediate temporary relief of symptoms can be achieved by administration of supplemental oxygen, systemic anticoagulation, diuretics, and corticosteroids.3 Radiation therapy or chemotherapy can effectively relieve the signs and symptoms of SVC syndrome by completely or partially shrinking the tumor, thereby restoring ante-grade blood flow through the SVC.13,14 Initial success rates have been reported to exceed 90%.15,16 In some patients, symptomatic relief may take up to 3 weeks with standard management.15 In 10 to 20% of treated patients, recurrence of SVC syndrome occurs as a result of tumor progression, tumor relapse, postradiation fibrosis, or venous thrombosis.6,16 Surgical management of patients with “malignant” SVC syndrome is associated with significant morbidity.17 Furthermore, many of these patients are poor operative candidates with limited life expectancy. Interventional radiologic techniques become the mainstay of therapy when “malignant” SVC syndrome is (1) slow to respond, (2) refractory to standard management, or (3) recurrent. Generally, thrombolytic therapy is reserved for cases of complete SVC occlusion.11 Interventional radiologic treatment of “malignant” SVC syndrome invariably involves the placement of metallic stents (Fig. 29–1). Angioplasty should be performed before stent placement to localize the point of maximal stenosis and to gauge the potential success of stent placement. If the lesion is so tight that it cannot be effaced by using a high-pressure angioplasty balloon, stent placement is contraindicated. Percutaneous placement of a stent to treat “malignant” SVC syndrome must be viewed as a palliative therapy aimed at resolving the patient’s symptoms. Since the first description of the technique by Charnsangavej et al in 1986, at least 28 clinical series have been reported in the literature.7,11,12,16,18,19 The vast majority of patients with “malignant” SVC syndrome treated by interventional radiologists have advanced disease and are usually refractory to standard management; therefore, the results of the reported techniques must be viewed accordingly. Outcomes usually are measured in terms of whether the intervention remains clinically successful at the time of the patient’s death rather than on long-term patency. Successful treatment in the clinical series, defined as complete or partial resolution of symptoms, was 68 to 100%.7 Laing et al12 reviewed the results of 190 cases of SVC stenting from clinical series published within the last 5 years.12 Significant improvement of symptoms of SVC obstruction was achieved in 174 (91%) cases, with primary and secondary patencies over the duration of follow-up of 78 and 84%, respectively. The most commonly used stents in these published series were the Gianturco Z stent (Cook, Bloomington, IN), the Palmaz stent (Cordis/Johnson & Johnson Co., Warren, NJ) and Wallstent (Schneider/Boston Scientific Corp., Minneapolis, MN). Primary and secondary patency rates achieved in 123 cases using Gianturco Z stents were 74 and 80%, respectively. Primary and secondary patency rates achieved in 61 cases using Wall-stents were 83 and 90%, respectively. Excellent results also have been achieved using Palmaz stents, but limited numbers have been reported. Although a concern, tumor ingrowth is a relatively minor problem in that most patients die of their underlying malignant disease before restenosis can be a significant problem. Thrombosis is the most common cause of stent failure and recurrence of symptoms. In published clinical series, stent thrombosis was observed in 0 to 45% of cases.7 This wide disparity is due to the fact that follow-up is typically short, and most patients die from their underlying malignancy within a few months of stent placement. This raises the question of prophylactic anticoagulation for treated patients. Some investigators believe that routine use and cost of long-term anticoagulation with warfarin is difficult to justify in palliative care of patients with a short life expectancy.12 Others have routinely used warfarin for 3 to 6 months after stenting.7,12 If the flow is brisk with a sufficient luminal diameter post stent placement, some authors advocate a daily enteric-coated aspirin, with or without ticlopidine, to prevent clot formation.7 A consensus for anticoagulation strategy in these patients does not exist, nor can it be inferred from the available clinical data. FIGURE 29–1. A 37-year-old woman with breast cancer and mediastinal adenopathy presented with superior vena cava (SVC) syndrome. Her symptoms did not improve with systemic anticoagulation. Bilateral upper-extremity venograms via basilic vein apporach (a) shows thrombosis of the right subclavian and both innominate veins with reconstitution of the SVC. Multi-sidehole infusion catheters were introduced, one through each arm, and positioned within the thrombus burden. Urokinase infusion was initiated at 80,000 U/h through each catheter with concurrent systemic heparinization. Venograms were performed after 12 hours (not shown) and after 22 hours of urokinase infusion (b), which shows clot dissolution and persistent areas of venous narrowings. These venous segments were dilated with 10-mm balloon percutaneous transluminal angioplasty (PTA) catheter. Post angioplasty, hand injection of contrast showed significant recoil significant recoil (not shown). Therefore 10 × 96 mm Wallstents were bilaterally placed into the axillosubclavian veins (c). Completion bilateral venogram showed patent central venous system with antegrade flow (d). The patient’s arm, neck, and face swelling improved. Other Malignant Diseases Apart from SVC syndrome, symptomatic “malignant” superior central venous stenosis or occlusion is rare. Clinical presentations vary according to the vein segment affected, but none is so dramatic as SVC syndrome. Nonetheless, interventional management of these lesions follows the same principles and techniques as for SVC syndrome of malignant etiology. For the purposes of this discussion, benign superior central venous stenoses and occlusions are subdivided into SVC syndrome, internal jugular vein stenosis and occlusion, subclavian-axillary vein thrombosis, central vein stenosis (e.g., catheter-related, hemodialysis-related), and other. Superior Vena Cava Syndrome Benign diseases account for 10 to 15% of the cases of SVC syndrome and include sclerosing mediastinitis (histoplasmosis, sarcoidosis, tuberculosis), substernal goiter, ascending aortic aneurysm, constrictive pericarditis, trauma, radiation therapy, and intravascular foreign-body–induced thrombosis (e.g., pacemaker leads, central venous catheters).12,20,21 The clinical presentation is indistinguishable from that seen in patients with SVC syndrome caused by malignant disease. Treatment of “benign” SVC syndrome is generally anticoagulation, with surgical management reserved for refractory cases. Surgical management for “benign” SVC syndrome entails the placement of an interposition spiral saphenous vein graft or synthetic conduit between the innominate or jugular vein and the distal SVC or right atrial appendage. Doty et al22 treated nine patients with “benign” SVC syndrome with a composite spiral graft constructed from the patient’s saphenous vein, which was split longitudinally and wrapped around a stent. They reported that seven of the nine grafts remained patent for up to nearly 15 years, and all but one of these patients currently remain symptom free. Del Campo and Casey23 reported the surgical placement of stented polytetrafluoroethylene bifurcated grafts in two patients with SVC obstruction extending into its major tributaries.23 Postoperatively, symptoms disappeared in one patient and abated in the other. Both grafts were occluded within 1 year; however, in the interim, the collateral venous circulation became well developed in both patients, enabling them to respond well to anticoagulation therapy. Interventional radiologic techniques are an alternative to surgery. If thrombus is present, it should be cleared first, preferably by thrombolytic therapy or using a mechanical device (this presently would constitute off-label use). The primary treatment of the underlying lesion is angioplasty using large-diameter balloons. Angioplasty has had disappointing results, with the best results seen in focal weblike stenosis rather than in long-segment stenosis.21,24–26 The failures were due to elastic recoil or restenosis. In addition, radiation-induced strictures and fibrotic strictures are resistant to angioplasty27,28 (Fig. 29–2). The role of stents in the treatment of benign lesions is less well defined. The published outcomes are limited to small series or anecdotal reports.7,12,21 All these had good immediate and short-term clinical success. Proponents of stenting argue that it does not preclude future surgical intervention. Currently, no recommendations can be made because randomized prospective clinical trials comparing interventional radiologic techniques to surgical management are lacking. Of note, stents with sufficient “hoop” strength should be used to overcome the inherent elastic recoil of these benign lesions. Cases of stent strut fracture, delayed stent migration, and stent embolization have been reported.18,29–31 Because of the potential of stent-associated thrombogenicity, most physicians recommend 6 months of warfarin therapy and a daily enteric-coated aspirin for life.7,21 Internal Jugular Vein Stenosis or Occlusion Isolated internal jugular vein stenosis or chronic occlusion is almost invariably due to catheterization. It is rarely symptomatic owing to the rich collateral drainage of the head and neck. The only indication for treating a focal stenosis or chronic occlusion of the internal jugular vein is to facilitate placement of a central venous catheter or hemodialysis catheter in a patient with limited access. After traversal of the stenotic or occluded segment, angioplasty is performed and followed by placement of the catheter.32 Catheter-related thrombosis of the internal jugular vein is usually asymptomatic unless complicated by sepsis, pulmonary embolism, or propagation into more central veins. Treatment consists of antibiotic therapy, anticoagulation, and removal of the catheter. With propagation of the thrombus into the innominate vein and SVC, the patient may present clinically with symptoms of either axillosubclavian vein thrombosis or SVC syndrome, respectively. Subclavian–Axillary Vein Thrombosis Thrombosis of the axillary–subclavian vein was described by both Paget in 1875 and Von Schroetter in 1884.33,34 Hughes35 coined the eponym Paget–Schroetter syndrome in 1949 after the review of 320 cases of upper extremity venous obstruction. Since then, several etiologic factors of subclavian–axillary vein thrombosis (SAVT) have been recognized (Table 29–2). Primary SAVT is idiopathic or related to physical activity or arm positioning with or without anatomic compression at the thoracic outlet.36 All other causes are referred to as secondary SAVT. Idiopathic primary SAVT is a diagnosis of exclusion. It is important that patients aged under 40 years presenting with SAVT should undergo a full coagulopathy workup to exclude antithrombin III, protein C, or protein S deficiency, as well as the presence of other hypercoagulable states (Tables 29–3 and 29–4).36 FIGURE 29–2. An elderly woman with lung cancer treated with radiation therapy developed late onset of superior vena cava syndrome with no measurable residual disease by computed tomography. She was felt to have superior vena cava syndrome due to radiation fibrosis. Superior vena cavography via a right internal jugular approach shows a high grade stenosis involving the right innominate vein and superior vena cava (a). The patient also had multiple calcified nodes in this region raising the possibility of fibrosing mediastinitis. This lesion was treated with two tandem Palmaz stents dilated to 12 mm initially and flared to 15 mm inferiorly. Post stent venogram (b) unsubtracted; (c) subtracted. Excellent result is seen. The patient became symptom free until her death 1 year later. Primary Anatomic venous compression at the thoracic outlet (Paget-Schroetter syndrome) Strenuous upper-body activity (effort vein thrombosis) Upper-limb immobility Secondary Central venous catheterization (catheters, ports) Hemodialysis conduits and fistulas Parenteral nutrition and infusate-related (sclerosants, vesicants) Pacemaker wires Intravenous drug abuse Local compression by tumor, metastatic disease, lymphadenopathy Radiation fibrosis Cardiac failure Shoulder trauma Amyloidosis Sarcoidosis Oral contraception Primary Subclavian–Axillary Vein Thrombosis Primary SAVT accounts for fewer than 2% of all cases of deep vein thrombosis (DVT).37,38 “True” Paget–Schroetter syndrome refers to primary SAVT related to a thoracic outlet abnormality, with the thrombotic event often precipitated by physical activity or arm positioning.36 There is extrinsic compression of the axillary–subclavian vein caused by narrowing of the costoclavicular space and by bony and soft-tissue structures, exacerbated by shoulder depression. The axillary vein is subject to compression between the pectoralis minor muscle and the rib cage, and the subclavian vein may be compressed as it passes between the clavicle/subclavius muscle anteriorly and the first rib/scalenus anticus posteriorly39–42 (Fig. 29–3). In addition, a cervical rib, congenital fibro-muscular bands, muscle hypertrophy, and callus from a healing calvicular fracture also may compress the axillary–subclavian vein.43–44 “Effort vein thrombosis refers” to primary SAVT caused by repetitive, stenuous activities.45 The diagnosis of idiopathic primary SAVT is one of exclusion. Regardless of the cause of primary SAVT, the clinical presentation is often one of dramatic onset in young, active, healthy persons, typically men (>70%) with an average age of 31 years.46,47 In more than 70% of cases, the dominant arm is involved and a history of unusual activity or positioning of the affected arm can be elucidated.46,47 The patients clinically present with swelling of the affected arm and pain or discomfort that is clearly related to the use of that arm.36 Dunant48 reported that up to 80% of patients presenting with SAVT have had previous symptoms of intermittent venous claudication. Other symptoms described are paresthesia and pruritis. Physical examination of the affected arm often reveals bluish discoloration, palpable tender veins, and superficial venous engorgement over the arm, shoulder, and chest wall. Elevation of the affected extremity and elastic support helps relieve the swelling and discomfort but will not control or prevent recurrent symptoms with arm activity.36,40,44,47 Standard anticoagulant therapy effectively reduces clot extension and risk of pulmonary embolism, but it does little to affect the long-term disability.49,50 The long-term disability is related directly to the length of the thrombosed vein segment and the subsequent level of arm activity.47 A minority of patients will have little or no further discomfort and swelling, possibly resulting from spontaneous recanalization (<10%), short segment thrombosis with well-developed compensatory collateral flow (<20%), or little continuing need by the patient to use the arm actively.47 Deficiencies of protein C, protein S or antithrombin III Factor V deficiency Elevated concentrations of factor VIII Antiphospholipid antibody syndromes and lupus anticoagulant Myeloproliferative disorders (e.g., polycythemia vera) Heparin-induced thrombocytopenia Elevated baseline serum activated partial thromboplastin time Lupus anticoagulant test Anticardiolipin antibody test Russell’s viper venom test The management of patients with primary SAVT is highly controversial, and no consensus exists on the optimum treatment strategy. Various treatment strategies, using interventional radiologic techniques, surgical techniques, or both, and their relative timing have been described.40,47,51 These studies are retrospective and generally include small patient numbers. No randomized, controlled trials comparing the various treatment strategies have been done. Nevertheless, most physicians direct initial treatment to the thrombotic occlusion, followed by correction of any extrinsic anatomic impingement and finally treatment of any underlying venous stenosis.39–42,52,53 Before institution of any therapy for SAVT, it is imperative that a complete evaluation of the venous system be performed to assess the location and extent of the thrombus burden as well as the extent of the collateral circulation. The most widely preferred treatment for primary SAVT is Machleder’s multidisciplinary approach.40,54 Removal of the thrombotic occlusion may be accomplished by surgical thrombectomy or thrombolytic therapy. Surgical thrombectomy is most effective when performed within 5 days of the onset of acute symptoms.45,47 Although surgical thrombectomy is effective in removing the clot, rethrombosis rates are high. Catheter-directed thrombolytic therapy with concomitant systemic heparinization is currently the preferred method for removing the clot.42,47,54 For primary SAVT, the efficacy of catheter-directed thrombolytic therapy drops significantly if instituted after 10 days of acute symptom onset.55,56 Successful catheter-directed thrombolytic therapy has been reported for DVT occlusions of the lower extremities of greater than 10 days’ duration.57 Once the thrombus has been cleared, venography should be performed with the arm in the neutral position and in stress positions (Fig. 29–4). These views will help to determine whether there is any extrinsic compression of the axillary-subclavian vein at the thoracic outlet. The commonly performed stress venographic views are with the arm abducted 90 and 180 degrees with external rotation of the shoulder (palms of hands facing up). If this fails to show any extrinsic compression, the military position can be tried, with the arm down by the side, palms facing up, and the shoulders rotated back or with the arm positioned in the manner that elicited the patient’s symptoms.36,47 It is important to remember that extreme hyperabduction of the arm can physiologically cause extrinsic compression or occlusion of the axillary-subclavian vein, even in normal patients.

 Malignant Disease
Malignant Disease
 Benign Disease
Benign Disease
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



