Fig. 19.1
Intraoperative photographs showing the cutdown technique of femoral cannulation in preparation for cardiopulmonary bypass prior to redo sternotomy. (a) Small groin incision is performed to expose the femoral vessels and Seldinger technique using a guidewire is performed with purse-string sutures on the vessels. (b) The puncture site is then dilated over the guidewire prior to insertion of the cannula. (c) The femoral arterial and (d) femoral venous cannulae are inserted and secured in place with the final position of the venous cannula confirmed using intraoperative transesophageal echocardiography. When the femoral artery is noted to be small, a chimney graft is sewn end-to-side to the femoral artery. One or both groins can be cannulated based on vascular anatomy. Frequent postoperative examination of distal pulses and extremity perfusion are essential to identify potential compartment syndrome
Currently, we use a percutaneous cannula placed into the right internal jugular vein and advance it into the superior vena cava for additional venous return.
Vacuum-assisted venous drainage is used selectively and is avoided if there are any residual intracardiac shunts so that if injury occurs to a cardiac structure (usually right-sided) during dissection, air embolism is avoided.
Certain situations may necessitate initiation of cardiopulmonary bypass (CPB) before sternotomy, and this is determined by the relationship of mediastinal structures to the sternum and chest wall (Fig. 19.2) and the number of previous sternotomies.
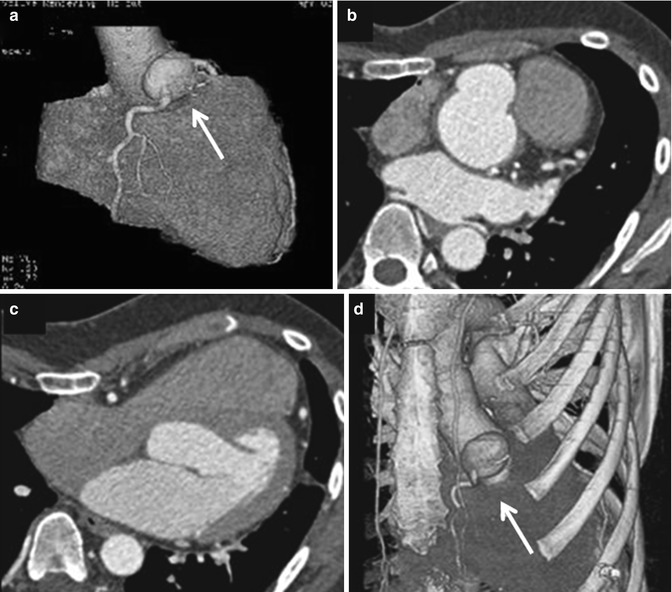

Fig. 19.2
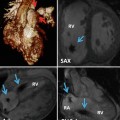
Preoperative cross-sectional imaging with 3-D reconstruction are of paramount importance in reoperations. (a) 3-D reconstruction showing a right coronary artery aneurysm (arrow); (b, c) the same patient also has a severe pectus deformity with the mediastinal structures displaced into the left hemithorax; (d) 3-D reformat showing the relationship of the leftward position of the right coronary artery aneurysm (arrow) to the sternum
The use of pericardial substitute (e.g., Gore-Tex pericardial membrane) may be considered if additional reoperation(s) is expected in order to minimize or reduce the risk of cardiac injury; however, their use has been controversial [5, 6]. A previous report from our institution identified that the incidence of injury is reduced if the native pericardium was approximated during the prior surgical procedure [4].
The initiation of CPB prior to resternotomy does not necessarily protect the right ventricle (RV) from injury; however, it provides a decompressed right heart and creates a controllable situation and minimizes the amount of blood loss if injury occurs and facilitates dissection. This is particularly helpful when there is significant right heart enlargement or there are elevated right-sided pressures.
Although CPB can facilitate a safe resternotomy, it does result in longer CPB times, which has been shown to increase mortality and morbidity. As a result, the operation should be orchestrated so that the CPB time is as short, but safe, as possible. This can be accomplished by using peripheral CPB to facilitate resternotomy, then separating from CPB after sternal division to complete the mediastinal dissection whenever possible. In some situations, the dissection may be facilitated if the heart is maintained decompressed while on CPB; the decision to perform the dissection on or off bypass is individualized.
Controversy exists regarding the need to remove the previously placed sternal wires or to retain them in place during the actual sawing of the sternum (Fig. 19.3a). There is no conclusive evidence that cardiac injury is decreased with keeping them intact; the decision is generally based on surgeon preference.
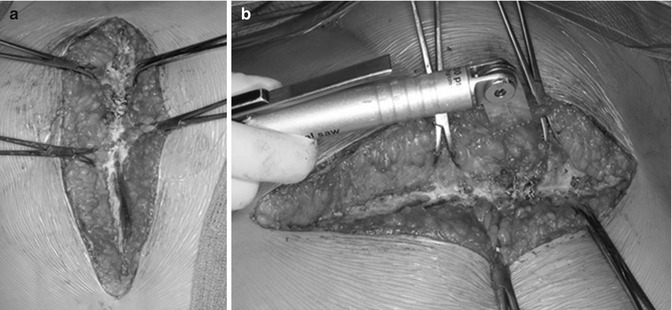

Fig. 19.3
Intraoperative photos demonstrating our technique of resternotomy: (a) towel clips are used on either side of the sternum to elevate the corresponding half during the resternotomy; (b) the microsagittal saw is preferred during redo sternotomy due to its ease of handling. In general, we use the saw for division of the anterior sternal table and heavy blunt scissors to divide the posterior table
We use the air-driven microsagittal saw (Fig. 19.3b) during repeat sternotomy and divide the anterior sternal table with the saw followed by careful division of the posterior table with a heavy scissors under direct vision with the help of Volkmann retractors that elevate the two halves of the sternum (Fig. 19.4a). The microsagittal saw has the advantage of precise perpendicular division of the sternum, with relatively easy control of the depth of blade penetration and the ability to feel the posterior sternal table [7].
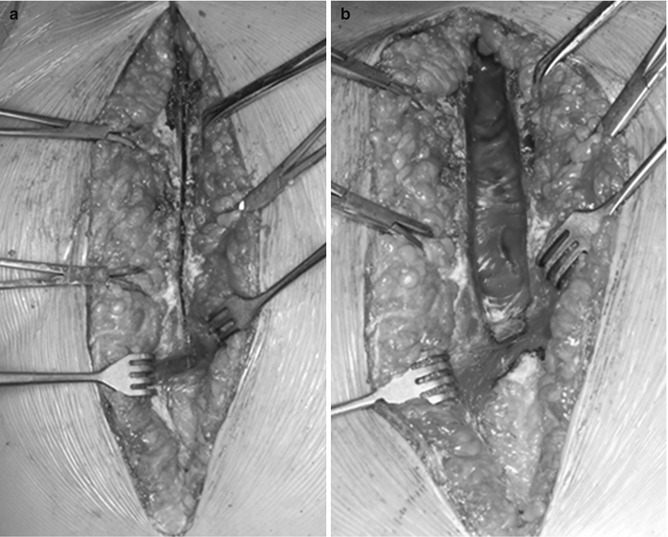

Fig. 19.4
Intraoperative photos demonstrating our preferred technique of resternotomy: (a) The anterior table of the sternum is divided with the microsagittal saw, and then with the help of Volkmann retractors, the posterior table is divided with the scissors under vision; (b) after dividing the posterior sternal table, the mediastinal structures are dissected and released carefully from the sternum using low-energy electrocautery and scissors. Each hemithorax is entered so the mediastinum falls away from the chest wall allowing sternal separation without tearing of upper mediastinal or cardiac structures
The heart and great vessels should be gently released in a stepwise fashion from the back of the sternum with combination scissors and low-energy electrocautery to allow safe separation of the two sternal halves and safe placement of the sternal retractor later on (Fig. 19.4b). We intentionally enter each pleural space so that the mediastinum falls away from the chest wall.
Limiting dissection only to the necessary areas of the intended procedure is an important consideration as it decreases the risk of injury to cardiac structures and results in less bleeding from dissected areas at the end of the procedure.
Role of Echocardiography
Echocardiography plays a significant role in the preoperative preparation for cardiac surgery, for both primary and repeat operations. It provides an accurate diagnosis, details about valve anatomy, the presence of septal defects, outflow tract anatomy, chamber dilatation or hypertrophy, and ventricular function. Preoperative transthoracic echocardiography and intraoperative transesophageal echocardiography are used routinely. Specific issues essential in the planning of a reoperation are outlined below:
It is important to identify an intracardiac shunt (atrial or ventricular level) as there is a risk of air embolism if inadvertent cardiotomy occurs while the heart is decompressed on cardiopulmonary bypass. When a septal defect is present, we maintain a positive central venous pressure of at least 5 mmHg to avoid air entry into the right heart with subsequent systemic embolization across the shunt. An aortic tack vent is used routinely and the bed is maintained in Trendelenburg position and the operative field is flooded with carbon dioxide. If the laceration involves a left-sided structure, aorta or pulmonary venous atrium, then CPB and deep hypothermia may become necessary to facilitate resternotomy.
Analysis of all intracardiac valve function is essential, but evaluation of the aortic valve to identify aortic regurgitation is particularly important. When aortic regurgitation is present, venting of the left ventricle is critical in the event that ventricular fibrillation occurs (which is expected with hypothermic bypass) in order to avoid distention of the left ventricle and its deleterious effects on ventricular function.
Intraoperative transesophageal echocardiography also provides guidance for peripheral cannulation and ensures proper positioning of both arterial and venous cannulae; it helps in evaluating collapse of the cardiac chambers after initiation of peripheral CPB, provides a continuous evaluation to the contractile status and distension of the ventricle(s), and guides the de-airing process at the conclusion of bypass.
Role of Cross-Sectional Imaging Computed Tomography (CT) Scan
The relationship and position of cardiac structures (chambers and great vessels) to each other and to the chest wall is essential when planning reoperation. Anatomic landmarks especially coronary arteries may be obscured, and an extracardiac conduit or dilated ascending aorta may become very close or even adherent to the undersurface of the sternum or chest wall (Fig. 19.5). In advanced cases, imaging of these anatomic structures has demonstrated erosion into the sternum, and this significantly increases the risk of resternotomy. Cross-sectional imaging provides a road map of these important anatomic findings that is essential in the preoperative evaluation. The indications for CT scan or MR imaging include a congenital diagnosis and prior surgery; information about great vessel anatomy (dilatation, stenosis, etc.) is particularly helpful. Advantages and disadvantages of CT vs MR are beyond the scope of this chapter and described elsewhere in this textbook. In general, MR is preferred when feasible since the exposure to radiation is reduced and the need for multiple studies over a lifetime is common in the patient population. The recent introduction of three-dimensional reconstructions is also invaluable in redo surgery as it provides the surgeon with an anatomic model of the heart and mediastinal structures, their relation to the sternum, location of extracardiac conduits, and anomalous course of major coronary arteries, and facilitates operative planning (Fig. 19.6).
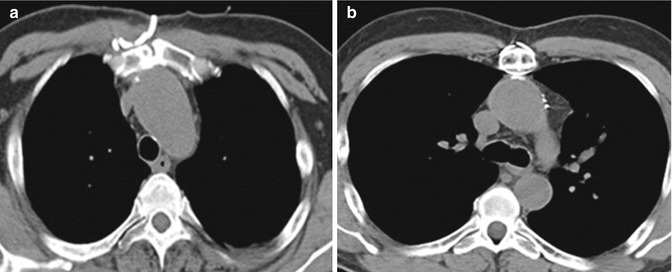
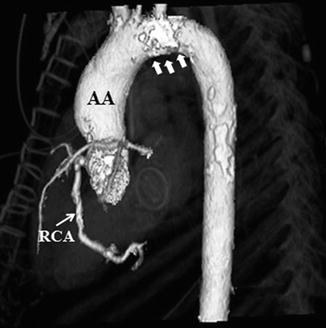

Fig. 19.5
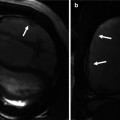
The value of preoperative cross-sectional images in demonstrating the relationship of the mediastinal structures to the underside of the sternum. In this particular case, the ascending aorta (a) and the aortic arch (b) are eroding into the sternum. Knowledge of this preoperatively is essential when planning redo sternotomy

Fig. 19.6
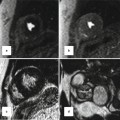
CT with 3-D reconstruction showing dilated ascending aorta (AA) above the sinutubular junction, location of the major coronary arteries in relation to the sternum, and the degree of calcification in the aortic arch (white arrows) and to a lesser degree in the ascending and descending aorta. RCA right coronary artery
Magnetic Resonance Imaging (MRI)
While MR imaging also provides excellent cross-sectional imaging similar to CT scanning, MR imaging is particularly helpful at providing additional information about cardiac chamber size and ventricular function. Decreased myocardial function has been one of the independent risk factors of perioperative mortality and overall outcome in many studies [8]. MRI plays an important role in evaluation of the right ventricular function which is difficult to evaluate by echocardiography with accuracy.
MRI is increasingly used in all types of patients with cardiac disease, including those with Ebstein malformation and other forms of congenital tricuspid regurgitation (TR) and right-sided disease (e.g., tetralogy of Fallot). Functional assessment can be made including quantitative measurement of left and right ventricular size and function (Fig. 19.7). At present, we rely on echocardiography (2- and 3-D) for detailed evaluation of valve anatomy (ventricular size and function to a lesser extent) and MRI for detailed assessment of right and left ventricular dimensions and function.
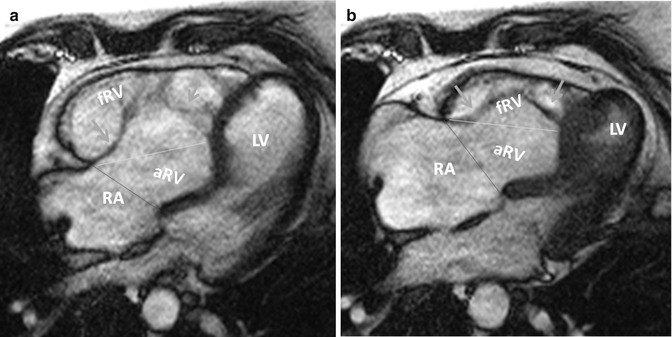

Fig. 19.7
Measurements of right ventricular volumes in Ebstein by MRI. Steady-state free-precession (SSFP) axial MR images in end-diastole (a) and end-systole (b) are shown. Tricuspid leaflets are shown by green arrows. The border between functional right ventricle (fRV) and atrialized right ventricle (aRV) is demarcated by yellow lines connecting the free wall attachment of the anterior tricuspid leaflet and septal attachment of the apically displaced septal leaflet. The border between the aRV and the right atrium (RA) is shown by red lines connecting the free wall attachment of the anterior tricuspid leaflet and the point of presumed normal attachment of the septal tricuspid valve leaflet. Measurements are obtained in end-diastole (a) and end-systole (b). The severity of clinical disease is thought to be inversely correlated to the size of the functional RV (fRV). In adults with unrepaired Ebstein anomaly, aRV volume is independently related to exercise test capacity and may express severity of disease [8]. LV left ventricle (Courtesy of Farhood Saremi MD. University of Southern California)
Role of Cardiac Catheterization
Cardiac catheterization is used selectively in the preoperative testing for adults with CHD prior to reoperation. Its role is mostly related to hemodynamic assessment in selected situations. However, cardiac catheterization can also play a supplemental role in imaging, e.g., great vessel anatomy, pulmonary artery, and coronary anatomy. Knowledge of coronary artery anatomy and distribution and the presence of acquired lesions are critical when planning resternotomy and the potential need for bypass grafting. Patients who had prior repair of tetralogy of Fallot with or without RV-to-pulmonary artery (PA) conduit may require reoperation for pulmonary valve or conduit replacement. Limited ventriculotomy may be necessary during pulmonary valve replacement or right ventricular outflow tract (RVOT) reconstruction. Accurate placement of this incision is of paramount importance to avoid injury to important coronary artery branches that may be crossing the RVOT (e.g., anomalous origin of left anterior descending branch from the right coronary artery) and this can be clearly delineated from preoperative cardiac catheterization or CT cross-sectional images as well (Fig. 19.8).
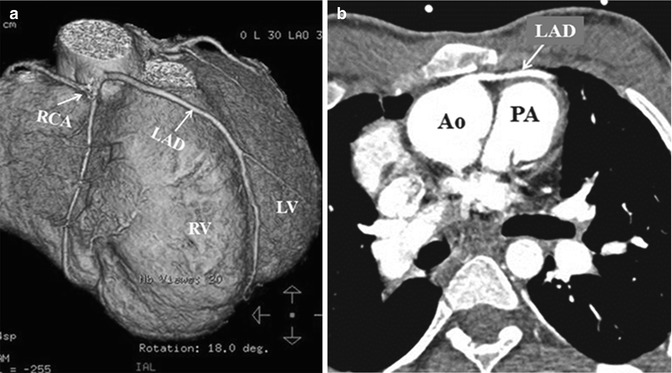

Fig. 19.8
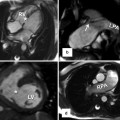
Computed tomography scan with 3-D reconstruction (a) showing: anomalous origin of the left anterior descending artery (LAD) from the right coronary artery (RCA) crossing the right ventricular outflow tract in a patient with tetralogy of Fallot. Corresponding cross-sectional image (b) shows its close proximity to the sternum. RV right ventricle, LV left ventricle, Ao aorta, PA pulmonary artery
Alternate Cannulation Sites and Peripheral Access Evaluation: Role of Ultrasonography
Patients with CHD frequently require repeat operations over a lifetime. As the number of sternotomies increases, the risk of cardiac injury increases as well. Peripheral vascular access may be compromised and limited from multiple prior cardiac procedures including open-heart surgery with perioperative invasive monitoring, cardiac catheterization(s), electrophysiology procedure(s), and/or pacemaker insertion. This may lead to scarring, stenosis, and obstruction of peripheral arterial and venous structures in the neck and groin, or they may be at risk for pseudoaneurysm, arteriovenous fistulae, or other complications associated with any of these procedures. Since peripheral cannulation for cardiopulmonary bypass becomes a strategic alternative and facilitates initiation of bypass prior to a hazardous resternotomy, knowledge of the peripheral vessel patency (groin and neck) is essential during the preop workup.
Preoperative evaluation of peripheral vasculature using ultrasonography is very important during the preoperative evaluation as it will change the plan with regard to cannulation. Peripheral arterial options include femoral or axillary artery; occasionally the innominate or carotid artery is considered in young children. Peripheral venous options include femoral and right internal jugular vein with advancement of the cannula into the right atrium via the superior or inferior vena cava. The decision to expose or cannulate the peripheral vessels and the timing of initiation of CPB before sternotomy is individualized, and preoperative imaging aids in this decision making.
The following alternative cannulation sites may be considered if there is a high risk of cardiac injury during the sternotomy:
Femoral artery or vein vessels
Iliac artery or vein vessels
Axillary artery
Carotid artery (usually right)
Innominate artery
Internal jugular vein (usually right)
Right atrium or ascending aorta (via right thoracotomy)
Left ventricular apex (via left thoracotomy)
Pulmonary artery (via left thoracotomy)
Abdominal aorta
Specific Pathology
The following lesions are examples of how preoperative imaging plays a critical role in guiding the surgeon during reoperation and minimizing the risks associated with resternotomy.
Tetralogy of Fallot/Pulmonary Atresia
Patients who had prior tetralogy of Fallot (TOF) or pulmonary atresia with ventricular septal defect (PA/VSD) repair using an extracardiac conduit, the conduit usually lies to the left of the midline and the aorta may be dilated (as is the case with all conotruncal anomalies) and they may be in close proximity to the back of the sternum (see Fig. 19.8).
Coronary artery anomalies are not uncommon and determination of the location of major coronary arterial branches (i.e., anomalous left anterior descending from the right coronary artery) is important during reoperation (Fig. 19.8). Anomalous vessels can be vulnerable during sternal reentry or at the time of any ventricular incisions that may be needed for a given procedure.
Truncus Arteriosus/Transposition of the Great Arteries
The Rastelli procedure (VSD closure with RV-PA conduit placement) has been the most common procedure to treat transposition with VSD and pulmonary stenosis. The position of the RV-to-PA conduit can be located to the left or right of the ascending aorta. Either way, the conduit commonly lies in a midline position and very close to the back of the sternum or even eroding into it.
Ross Procedure
This group of patients may have aneurysmal dilatation of the proximal aortic root – the pulmonary autograft (neoaorta). It is not uncommon for this proximal dilated segment of aorta to be abutting the sternum (Fig. 19.9




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree