Isotope
Compound
Half-life (days)
b-energy (MeV) (maximum)
g-energy (MeV) (%)
Soft tissue range (mm) (maximum)
32P
32P-phosphate
14.3
1.71
–
8
89Sr
89Sr-chloride
50.5
1.46
0.91 (0.01)
2.4
186Re
186Re-HEDP
3.7
1.07
0.137 (9)
2.4
188Re
188Re-HEDP
0.7
2.12
–
3
153Sm
153Sm-EDTMP
1.9
0.81
0.103 (28)
0.6
177Lu
177Lu-EDTMP
6.7
0.497
0.208 (11)
–
117mSn
117mSn-DTPA
13.6
–
–
Emits conversion electrons: 0.3
Mechanism of Action
32P-Sodium ortophosphate and 89Sr-chloride have direct affinity for bone. On the other hand, 153Sm, 186Re, 188Re, 177Lu, and 117mSn are targeted to bone by complexing the radionuclide to phosphonate complexes like EDTMP (lexidronam) or HEDP (etidronate). The sources of radiation within bone differ with the radiopharmaceutical used. The metallic chelated radiotracers tend to chemically absorb to the trabecular surface, whereas 32P-sodium orthophosphate and 89Sr-chloride distribute more widely throughout bone because of the natural distribution of phosphate and the fact that strontium behaves very much like calcium in the body, both being members of Periodic Table Family II. Due to the heterogeneity of radiopharmaceutical uptake, spicule thickening, and tumor and marrow distribution, there is variation in dosimetry [40, 45–49].
32P-Sodium orthophosphate
32P was the radionuclide initially used in the treatment of bone pain and remained the most widely used agent until the 1980s [47, 48]. It is a reactor-produced, pure beta-emitting radionuclide with a physical half-life of 14.3 days and emission of an energetic beta particle of 1.71 MeV. Its high energy allows a maximum tissue range 8 mm and an average range of 3 mm. It is available in forms in both oral and injectable forms. The mechanism of action appears to be due to DNA damage from decay to 32S with subsequent tumor cell death. Its uptake in malignant tissue was demonstrated by Lawrence and colleagues [49].
The response rates for 32P therapy were between 59 and 93% for prostate cancer and 52–94% for breast cancer with overall response rates of 77% and 84%, respectively [50–52]. No dose–response relationship was seen. Pain palliation is seen usually within 14 days with a range of 2 days to 4 weeks. The long beta particle range resulted in significant radiation to the bone marrow, with frequent hematopoietic side effects; however grade 4 leukopenia and thrombocytopenia are rare [48]. Dose-dependent pancytopenia occurs at around 4–5 weeks with recovery by 6–7 weeks.
89Sr-Chloride
89Sr-chloride is one of the two FDA-approved radiopharmaceuticals that has largely replaced 32P-sodium orthophosphate in the treatment of metastatic bone pain. It belongs to the II-A family of the Periodic Table of elements and is similar to calcium in its biological behavior. It is administered intravenously and is rapidly concentrated in the bones, mainly in osteoblastic areas. 89Sr has a physical half-life of 50.5 days and emits a beta particle with a maximum energy of 1.46 MeV, with a maximum beta particle range of 7.2 mm and an average soft tissue range of 2.4 mm. It has very low gamma emissions (0.01% abundant gamma emission with a 0.91 MeV photopeak) precluding external imaging. About 80% of free 89Sr is excreted through the kidneys and about 20% through the gastrointestinal system. Its biological half-life is 4–5 days. There is evidence of biexponential excretion, such that 20% remains in the body, mostly in bone, at 90 days [53].
The administered activity is generally 40 μCi/kg or 4 mCi. The onset of pain relief commonly occurs within 7–21 days, and mean duration of relief is about 2–6 months. Transient increased bone pain (painful flare) may occur in about 10–15% of patients and is usually seen within 24–120 h posttreatment. This is usually mild, self-limited, and controlled with analgesics. A flare also usually heralds a good treatment response [54]. Hematologic toxicity is usually mild and reversible. There is a minimal decrease in red-cell counts. In various studies, platelet and white cell counts may drop on an average by 30–40% from the pretreatment level, though nadirs as low as 25–30% of pretreatment values have been seen [40, 55–57].
Re-treatment can be given after 90 days, however detailed reassessment and close follow-up is recommended because multiple therapies may lead to greater marrow toxicity. 89Sr-chloride has been extensively used, mainly in the treatment of bone pain from breast and prostate cancer, with 60 and 80% of patients obtaining some relief of bone pain [40, 54, 58, 59]. Repeated therapy with 89Sr-chloride is effective in relieving pain, though the response rate and duration of response may be lower than with first treatment [60–62].
89Sr-chloride therapy given concurrently with chemotherapy may enhance pain relief and delay the onset of new painful metastases [63]. Response rates (complete plus partial response) of 55–80% have been seen with 89Sr-chloride when used in combination with chemotherapy. In a randomized trial in patients with hormone refractory prostate cancer, cisplatin administered with 89Sr-chloride showed better overall pain relief (91% vs. 63%), longer median duration of pain relief (120 days vs. 60 days), lower incidence of new sites of pain (14% vs. 30%), and lower progression of bone metastasis (27% vs. 64%) as compared to those with 89Sr-chloride and placebo, respectively. However, there was no significant difference in survival or toxicities between the groups.
Similar findings were noted by Quilty et al. comparing 89Sr-chloride with radiation therapy in patients with prostate cancer [64]. There was longer time to onset of new painful sites in those treated with 89Sr-chloride than in those who received radiation therapy alone, and fewer patients reported new pain sites after strontium therapy than after local or hemibody radiotherapy in pain palliation for prostate cancer. However, there was no survival benefit [65, 66] even after using a higher activity (10.8 mCi).
Combined 89Sr-chloride and chemotherapy may have survival benefit in androgen-independent prostate cancer. Significant increase in median overall survival was seen in those who received 89Sr and doxorubicin (27.7 months) compared to those who received doxorubicin alone (16.8 months) [67]. 89Sr-chloride is also cost effective, as the need for radiotherapy narcotic analgesic use and hospitalization is reduced [41, 68].
In breast cancer patients with bone metastases, Sciuto et al. observed pain relief in 84% of patients with duration of pain relief ranging from 2 to 14 months [69]. Other studies in breast cancer have also reported pain relief in about 60–80% of patients with duration of the response between 3 and 12 months. Good relief (in 83% of patients) was also seen in patients retreated with 89Sr-chloride [70, 71]. Responses are better in patients with osteoblastic lesions, in those with limited involvement and higher performance status [72].
153Sm-EDTMP (Lexidronam)
153Sm is a fission product with a half-life of 46.3 h. The radionuclide emits a beta particle with a maximum energy of 0.81 MeV, a mean energy of 0.23 MeV, an average soft tissue range of 0.6 mm and a 28% abundant gamma emission with a photopeak of 0.103 MeV. 153Sm-EDTMP is usually administered at an activity of 1 mCi/kg. Gamma emissions of 153Sm allow gamma camera imaging to confirm the targeting of lesions [73, 74] as demonstrated on bone scans.
153Sm-EDTMP complex is made by complexing 153SmCl acid with the calcium salt of EDTMP. It is a stable complex that selectively accumulates in skeletal tissue in association with hydroxyapatite. It accumulates in high concentration in areas of bone high turnover like bone metastases [73, 74]. Bone metastasis may contain 3–5 times more 153Sm-EDTMP than healthy bone tissue and hence the amount of drug retained in the body is related to the extent of metastases not to the administered activity [74]. About 50–60% of the administered activity localizes to bone in 2–3 h with 30–50% excretion in urine, with larger amounts of activity deposited in bone in subjects with impaired renal function [74] or with widespread metastases.
153Sm-EDTMP is cleared rapidly from the blood with 4–34% of the activity remains in the serum 1 h after administration [75]. There is an initial rapid phase followed by a slower second phase with βT1/2 that may range from 8.1 to 17.1 h [74, 75]. Urinary excretion is the main route of elimination with majority of activity cleared at 6–8 h [74, 75].
The low energy of the beta particle reduces the radiation burden to bone marrow. Hematological side effects are generally mild. In an early study of dose escalation, Farhangi and colleagues studied 22 patients, giving escalating activities of 153Sm-EDTMP, from 0.1 to 1.0 mCi/kg (3.7–37 MBq/kg) [75]. The therapy was well tolerated with transient grade 1 myelotoxicity observed in 34.5% cases; those with extensive metastases were more likely to have myelotoxicity. A later study in 52 patients used escalated activities from 0.5 to 3.0 mCi/kg (18.5–111 MBq/kg) [58]. Treatment at higher doses was also well tolerated and maximum tolerated dose (MTD) was determined to be 2.5 mCi/kg (92.5 MBq/kg). Bone marrow suppression is generally mild, reversible, and not associated with grade 4 toxicity. Platelets and leukocyte may decrease about 10–40% from the pretreatment values generally reaching nadir at 3–4 weeks. The recovery of both is generally complete, reaching the pretreatment values by 6–8 weeks [40].
Multiple studies have shown the efficacy of 153Sm-EDTMP therapy in pain palliation with the average response between 62 and 74% [76, 77]. A statistically insignificant trend toward improved survival was seen in those treated with higher dose than those treated with lower activity [76, 78]. Placebo-controlled studies have shown significant pain reduction with 153Sm-EDTMP in a variety of cancers including hormone refractory prostate cancer [79]. 153Sm-EDTMP showed an improved overall efficacy in pain relief of 77.8% compared to 44.4% with pamidronate alone, though transient myelotoxicity was also higher [80]. It has been suggested that prior treatment with pamidronate does not affect 153Sm-EDTMP skeletal uptake [81].
Pain relief is generally noted within 5–10 days and can last up to 4 months. Repeated administration of 153Sm-EDTMP is feasible if pain returns or increases [82]. A phenomenon of transient exacerbation of the preexisting pain (flare) after 153Sm-EDTMP has been seen in 12–20% of patients [83]. A change in whole body uptake values for 99mTc-MDP on follow-up scanning after 153Sm-EDTMP therapy may possibly be seen [74], though some studies suggest no change in bone scan appearance [75].
Rhenium-Labeled Radiopharmaceuticals: 186Re-HEDP and 188Re-HEDP (Etidronate)
186Re and 188Re are two isotopes that are linked to bisphosphonates which have been tested in patients, but have not gained regulatory approval for clinical use in the USA or the European Union [84]. 186Re has a physical half-life of 3.7 days and emits a beta particle with a maximum energy of 1.07 MeV, a mean energy of 0.349 MeV, and an average range of 0.5 mm in dense bone and soft tissue range of 1.1 mm. It has some gamma emission of 137 keV, at low abundance (9%) that allows imaging. 186Re is conjugated to 1-hydroxyethylidene-1,1-diphosphonate (186Re-HEDP/186 Re(Sn)-HEDP or etidronate) complex, which attaches to hydroxyapatite crystals by forming hydroxide bridges.
186Re-HEDP has a plasma half-life of 41.0 ± 6.0 h [84] and about 70% of the activity is excreted in the urine within 24 h in patients free of bone metastases. The maximum tolerated activity is 2,960 MBq in patients with metastatic prostate cancer. The bone marrow absorbed dose is about 1·04 mGy/MBq [85]. A number of initial studies reported safety and efficacy of use of 186Re-HEDP in bone pain palliation. A study by Sciuto et al. with 186Re-HEDP showed an overall pain palliation rate of 92% in breast cancer patients [69]. Pain relief was established early and tumor/bone marrow absorbed dose ratio was 14:1, suggesting its clinical relevance in patients with more compromised bone marrow reserve. Typical marrow recovery times range from 4 to 6 weeks.
188Re is a generator produced isotope that may be “milked” on demand from a 188W/188Re generator. A kit is available for conjugating it to HEDP. 188Re has a beta energy of 2.1 MeV with a gamma emission of 155 keV (15% abundance) that allows for imaging. The high beta energy has the potential of killing tumor cells. The physical half life is 16.8 h. 188Re-(Sn)HEDP has similar biodistribution as 186Re-(Sn)HEDP. It shows rapid urinary excretion with 60 ± 12% of administered activity in urine by 48 h which varies with renal function and extent of osteoblastic metastases [86, 87].
There is limited data on the use of this agent in the treatment of bone pain. An initial study demonstrated pain palliation in 60–75% of prostate cancer patients receiving an activity of 2.6 GBq or higher [88]. The possibility of repeated therapy at low cost has led to the agent being investigated for use in developing countries. Recently, the effectiveness of repeated therapy using 188Re-HEDP has been demonstrated in 64 patients with progressive, hormone-resistant prostate cancer [89].
117mSn-Pentetate and 177Lu-EDTMP
117mSn-pentetate emits conversion electrons with therapeutic potential; their high LET and very short range in soft tissue (0.2–0.3 mm) may explain why there appears to be less myelosuppression with 117mSn-pentetate than with other agents. 117mSn is injected as the pentetate (DTPA) chelate and has no affinity for hydroxyapatite. The mechanism of localization is postulated as precipitation of stannous oxide on bone surfaces or by a hydrolysis reaction with hydroxyapatite. Research studies show that the pain palliation rate is approximately 75%. There is no dose–response relationship; at activities more than 12 mCi (per 70 kg body weight), pain palliation has been noted as early as less than 1 week after treatment [90]. 117mSn-DTPA is not approved for clinical use.
177Lu-EDTMP has been explored as a potential radiopharmaceutical for bone pain palliation [91]. 177Lu has a half-life of 6.71 days and is both a beta- and gamma emitter. Its role is not yet fully established.
Patient Identification and Referral
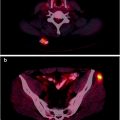
Patients are generally referred for radiopharmaceutical bone pain palliation by medical or radiation oncologists. Patients most commonly referred for bone pain therapy are those with breast and prostate cancer since osseous metastases are common and the role of radiopharmaceutical therapy is well documented for these cancers. An ideal patient is one with multiple painful bone metastases confirmed by a bone scan which shows multiple focal sites of increased radiopharmaceutical uptake and who is experiencing worsening of bone pain, in spite of narcotic pain medication.
Prior to radionuclide therapy, it is helpful to have a checklist to assure appropriate identification of the patient, confirm the patients clinical status, determine their contenance of urine/feces, corroborate the presence of bone metastases on bone scan (performed within 8 weeks), and determine adequate marrow reserve and renal function with a complete blood count and BUN/creatinine within 1 week. The patients name, medical record number, and date of birth should be confirmed. The clinical status should establish a life expectancy >60 days and the bone scan should demonstrate skeletal metastases involving multiple sites. As per SNM guidelines, the platelet count should be above 60,000/μL and the total leucocyte count above 3,000/μL—with an absolute neutrophil count (ANC) >2,000/μL (SNM practice guidelines. www.snm.org) The creatinine should be <1.5 mg/dL. Patients with an elevated creatinine are likely to have a prolonged biological clearance of the radiopharmaceutical, potentially increasing uptake in bone—and exposure of bone marrow. When the CBC and/or creatinine are outside these limits, the patient may be considered for reduced dose therapy or radiopharmaceutical therapy may be inappropriate.
A detailed history should be taken to evaluate other systemic illness like renal failure. All symptoms should be assessed to exclude acute cord compression as this emergency warrants immediate radiation therapy and/or sometimes surgery. Radiopharmaceuticals have no place in the management of spinal cord compression or in treating pathological fracture (Tables 27.2 and 27.3).
Table 27.2
Common indications and contraindications
Indications: |
Multiple painful osseous metastases |
Contraindications: |
Pregnancy; breast-feeding |
Acute/chronic renal failure |
Acute spinal cord suppression—needs external radiation therapy |
Hb < 10.0 g/dL |
Total white cell count < 3.0 × 109/L |
Granulocytes < 2,000/μL |
Platelets < 60 × 109/L |
GFR < 30 mL/min |
Table 27.3
Checklist prior to radiopharmaceutical administration
Positive bone scan (within 8 weeks) with abnormal uptake corresponding to pain sites |
Worsening bone pain despite pain medication |
No chemotherapy, external beam radiation, or treatment with bisphosphonates during the 6 weeks before therapy with agent |
Labs: Hb > 10.0 g/dL White cell count > 3.0 × 109/L Granulocytes > 2,000/μL Platelets > 60 × 109/L |
Administration of the Radiopharmaceutical Agent
Once the eligibility is established, the patient is usually treated in an outpatient setting. Patient identifiers are checked. Informed consent is obtained for administration of systemic radiopharmaceutical therapy after the possibility of transiently increased pain due to flare response is discussed. An intravenous line is inserted and checked for good blood return and free flow of the saline flush. Care must be taken to prevent extravasation of tracer which may cause tissue necrosis. Patient weight is checked to calculate the activity of 153Sm-EDTMP. 89Sr-chloride therapy is usually a flat activity of 4 mCi. The radiopharmaceutical is administered as a slow intravenous injection over a period of 1–2 min and flushed with 10–20 mL of saline.
In the USA, use and administration of these radiopharmaceuticals falls under the guidelines of nuclear regulatory commission (NRC), Title 10 CFR Part 35.300 or in agreement with state institutional license. Radiation level in the patient at surface and 1 m is checked. The patient is instructed about the radiation safety precautions (Table 27.4). For those administering the dose, contamination control involves employing universal body fluid precautions when working with the patient. Federal guidelines [92] do not require patient-specific ALARA (as low as reasonably achievable) instructions for patients receiving up to 140 mCi of 153Sm-EDTMP. In addition, there is no limit placed on patients receiving the pure beta emitter 89Sr because of the minimal exposure to members of the public. The nuclear medicine physician or a radiation safety officer typically discusses detailed radiation safety precautions after reviewing individual patient specifics.
Table 27.4
Some suggested radiation safety specifications discussed with the patients needing beta-emitting therapy for painful bone metastases
1. The radiopharmaceutical is removed from the body primarily through the urine, so practice good hygiene when using the toilet: Sitting down to urinate to reduce the possibility of contamination Flush toilet twice after use Wipe any spilled urine with a tissue and flush it away Avoid soiling underclothing or areas around toilet bowls Wash hands thoroughly after urination or cleaning the toilet |
2. Wash clothes and bed linens if they become soiled with urine or blood. Wash them separately from other clothes |
3. If cut, wash away blood. Wipe spilled blood with tissue and flush it away |
4. There is generally no medically significant exposure to other people and no requirement for isolation or limiting interaction with others |
5. Eat a normal diet |
Follow-Up
The patient usually follows up with the referring physician who evaluates the patient’s clinical response and obtains blood counts at recommended intervals (at least weekly for the first 6–10 weeks and more frequently if there is evidence of toxicity, although a drop in counts is uncommon before 7–14 days after treatment). Blood counts, notably platelet and white blood cell counts, should be obtained until all counts are normal; return to pretreatment baseline may not always occur.
Summary
Bone pain palliation therapy with radiopharmaceuticals is a cost-effective systemic therapy to relieve pain from skeletal metastases with a consequent decrease in morbidity and an improvement in quality of life. Bone-seeking radiopharmaceuticals as part of a multimodality regimen may also perhaps slow disease progression. Radiopharmaceuticals should be considered in patients with widespread osteoblastic metastases causing multiple painful skeletal sites. The ability to use this treatment with other therapies with added benefit is definitely an asset. While a single injection of such a radiopharmaceutical has been shown to be effective especially in metastatic prostate and breast cancer, repeat therapies should be carefully administered at a time when the blood counts have returned to safe levels are noted above. Although more than one radiopharmaceutical treatment for bone pain can be given, the white blood cell/platelet count in recovery eventually do not return to baseline levels. There are no blinded comparative studies showing statistically significant differences in efficacy, toxicity, or duration of response between these various radiopharmaceuticals.
Metastatic Bone Therapy
Combining Radiopharmaceuticals with Chemotherapy
In patients with established bone metastases, hormonal or chemotherapy can palliate symptoms, but rarely produces durable control. These findings suggest that tumor cells with the ability to metastasize to bone are intrinsically resistant, or as a result of continued exposure to factors in the bone microenvironment, are uniquely capable of surviving treatment. It is now believed that the stroma in the marrow secretes factors that enhance the survival of cells in the skeleton, contributing to treatment resistance.
For metastatic prostate cancer, initial therapy for metastatic disease involves androgen withdrawal, to which most patients respond and then relapse within 1–2 years. For patients who relapse despite androgen withdrawal, taxane-based chemotherapy can confer significant clinical benefits. Two randomized controlled clinical trials have demonstrated that docetaxel can prolong survival and enhance quality of life relative to a previously used regimen, mitoxantrone and prednisone [93, 94]. This survival benefit is only on the order of 2–3 months, however, likely because patients progress after 4–6 months of treatment with docetaxel.
Two bone-seeking radiopharmaceuticals, 89Sr-chloride and 153Sm-EDTMP, are presently approved by the FDA for the treatment of prostate cancer patients with painful bone metastases. Radiopharmaceuticals may have a role beyond the palliation of pain, however. Bone-seeking radiopharmaceuticals have been shown to slow the development of new metastatic sites [65]. In addition, in a recent randomized phase II trial in which patients either received doxorubicin alone or doxorubicin with 89Sr-chloride patients who received the combination appeared to enjoy an improvement in both disease-free and overall survival [67]. These data offer a compelling rationale to combine contemporary taxane-based chemotherapy with bone-seeking radiopharmaceuticals.
Studies have shown the feasibility of coadministering bone-seeking radiopharmaceuticals and common prostate cancer chemotherapy regimens, such as estramustine and vinblastine [95]. A small phase III trial was conducted in which patients were randomized to either 89Sr-chloride and cisplatin or 89Sr-chloride alone. Only 35 patients were on each arm. Chemotherapy enhanced the palliative effects of strontium (91% vs. 63%, p < 0.01) and increased the duration (134 days vs. 68 days, p = 0.002) of these effects. In addition, a lower proportion of patients who received both therapies progressed in bone than in those who received strontium only (27% vs. 64%, p = 0.01). However, significant alterations in prostate-specific antigen (PSA) were not seen and there was no significant difference in survival, which is not surprising given that cisplatin as a single agent is not active in prostate cancer and given the small size of the study.
Combinations of active chemotherapy with bone-seeking radiopharmaceuticals have had significantly more promising results. In a randomized phase II trial conducted at MD Anderson, 103 patients received induction chemotherapy using ketoconazole and doxorubicin alternating with estramustine and vinblastine. Patients who did not progress following induction were randomized either to 89Sr-chloride with doxorubicin or to doxorubicin only. Patients who received the bone-targeted therapy had a 14 month progression-free survival as opposed to a 7 month progression-free survival achieved by the patients who received chemotherapy alone. More important, patients treated with the 89Sr-chloride achieved an overall survival of 27.7 months as opposed to 16.8 months for those who received chemotherapy alone [67]. Although the trial was not powered to make direct comparisons of the two arms, the data do suggest that combinations of chemotherapy and treatments directed at bone metastases should be pursued.
Emerging data has suggested the usefulness of radiopharmaceuticals beyond pain palliation. In a small study, a single administration of 153Sm-EDTMP of 37 MBq/kg (1 mCi/kg) and weekly infusions of 35 mg/m2 docetaxel were given in 12 patients. With a mean follow-up was 11.4 months (range, 1.1–25.8), overall 1-year survival was 48.6%, and median survival was 11.5 months. A PSA response of >50% was seen in 50% of patients and pain relief seen in 58.3% of patients [96].
In a phase I study at MSKCC, patients were treated with combination of 153Sm-EDTMP and Docetaxel. Patients were assigned to six different cohorts based on increasing doses first of docetaxel and then of 153Sm-EDTMP. For cohorts 1–5, one cycle consisted of docetaxel and 153Sm-EDTMP followed by docetaxel alone 3–4 weeks later, followed by a 3- to 5-week observation period. Patients in cohort 6 received a third docetaxel administration 3–4 weeks after the second dose during each cycle. Cycle 1 toxicity was used to define dose-limiting toxicity (DLT); however, all cycle toxicities were recorded. The starting dose of docetaxel was 65 mg/m2, well within therapeutic range [97], whereas the starting activity of 153Sm-EDTMP was 0.5 mCi/kg, half the US Food and Drug Administration-approved dose for pain palliation. Docetaxel dose was escalated to 75 mg/m2, after which 153Sm-EDTMP was escalated to 1.0 mCi/kg. Grade 3–4 neutropenia for more than 7 days despite growth factor support or grade 3 thrombocytopenia triggered a 25% dose reduction of docetaxel and 153Sm-EDTMP in subsequent treatment cycles. Dose reductions were imposed for both drugs in the event of other serious hematologic or nonhematologic toxicities. Patients were taken off the study if they required more than two dose reductions, experienced grade 3 or 4 nonhematologic toxicities, or had grade 2 events that failed to resolve within 2 weeks. Twenty-eight men were treated in six cohorts. Maximum-tolerated dose was not reached, because full doses of both agents were well tolerated, even using an every-6-week dosing schedule of 153Sm-EDTMP. Patients received an average of 5.6 docetaxel doses (range, 1–13 doses) and 2.9 153Sm-EDTMP doses (range, 1–6 doses). Fifteen patients demonstrated a more than 50% decline in PSA. Treatment significantly reduced indices of bone deposition and resorption.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree