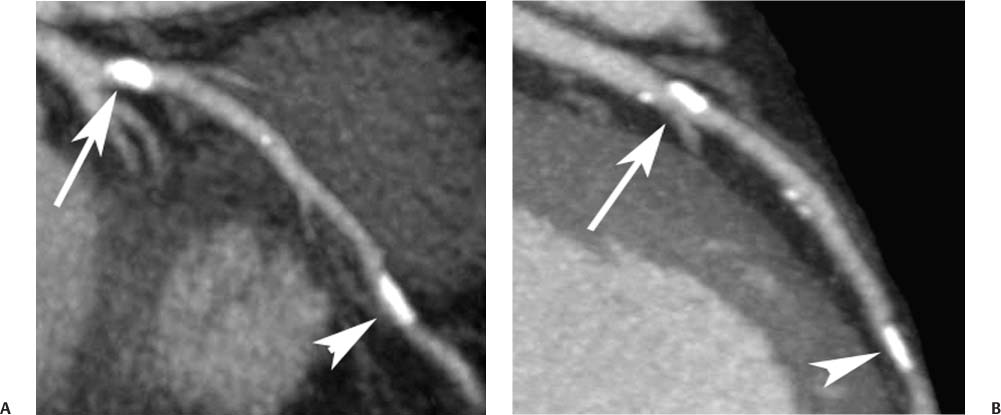
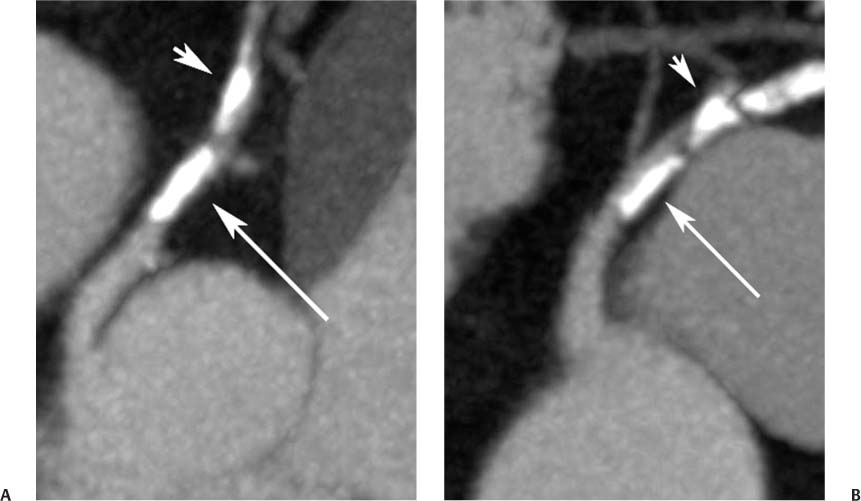
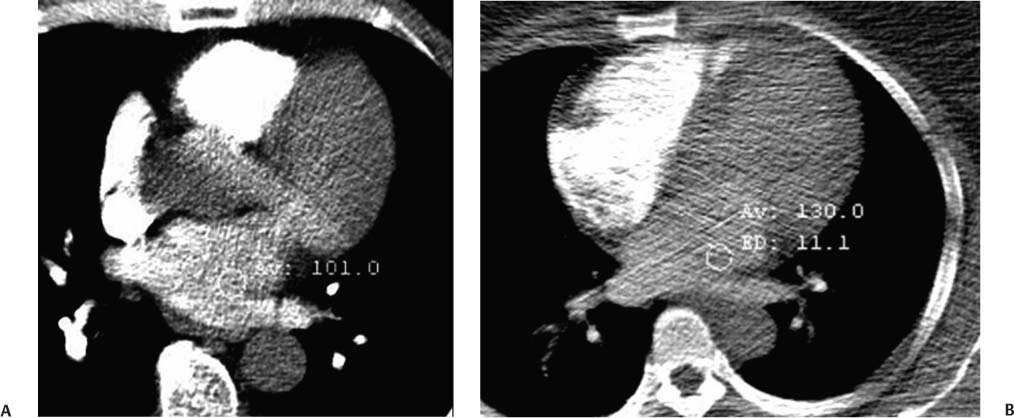
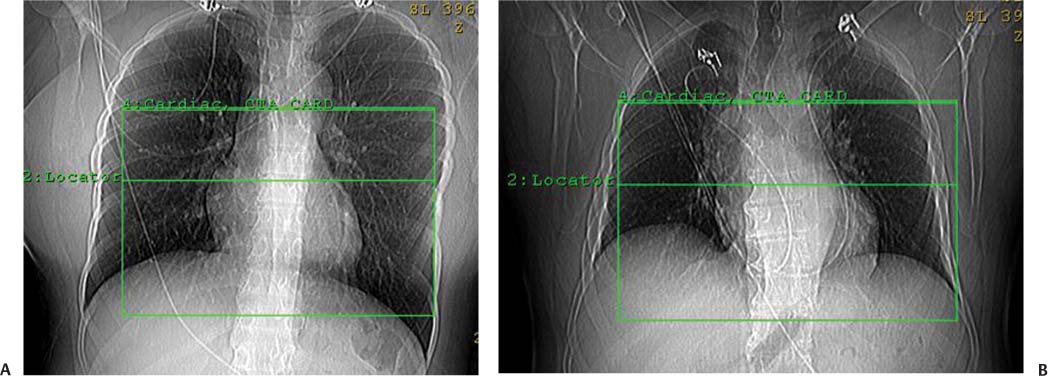
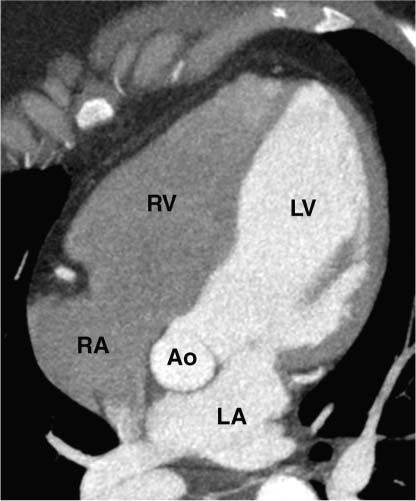
2 Technique is the major determinant of image quality for cardiac CT. Patient preparation before the examination, coaching of the patient during the examination, and appropriate CT technique are critical to image quality. Postprocessing techniques may enhance the diagnostic information present in a suboptimal examination, but they cannot compensate for information that is lacking in an improperly performed examination. This chapter addresses technical aspects of the cardiac CT evaluation, including those aspects of imaging physics relevant to optimization of image quality and reduction of radiation dose—the practical “how-to” guide for cardiac CT. A high-quality cardiac CT begins with proper patient preparation. To minimize ectopy in the cardiac rhythm, patients are asked to refrain from stimulants such as caffeine on the day of the examination. Solid foods should not be ingested for 4 hours before the study to reduce the risk of emesis. Liquid intake (water, milk, or juice) is encouraged up to 1 hour preceding the examination to maintain a well-hydrated patient before the administration of contrast. A patient interview before the CT examination should include questions about prior treatment and/or interventions for coronary disease. The presence of bypass grafts or stents will impact the protocol used for the study. If the patient has a paced heart rhythm, temporary reprogramming of the pacemaker to a slower rate may be helpful to obtain high-quality images of the coronary arteries. Each patient is questioned for a history of asthma, allergy to iodinated contrast material, or any history of a severe allergic reaction. β-blockers should be used with extreme caution in any patient with a history of asthma requiring treatment. Patients with a history of allergy to iodinated contrast should be pretreated with steroids to reduce the risk of an allergic reaction. A history of an anaphylactic reaction to iodinated contrast or other medication should serve as a red flag; contrast-enhanced studies should be performed in these patients only as an option of last resort. The protocol for steroid pre-medication at Thomas Jefferson University includes pretreatment with oral Medrol (methylprednisolone), 32 mg given 12 hours before the procedure and repeated again 2 hours before the procedure. Adequate intravenous (IV) access is necessary to ensure delivery of a rapid, tight contrast bolus. An 18-gauge IV line in the antecubital fossa is preferred, although a 20-gauge line may suffice. Once the IV access is placed, the line should be tested with a rapid hand injection of saline to ascertain that there is no extravasation and that the patient does not experience pain with injection. The test injection of saline should simulate the rate of 5.0 to 5.5 mL/sec that will be required for the subsequent injection during CT angiography (CTA). Injections performed at this rate are often pressure limited when the IV line is smaller than 18 gauge. To optimize the IV flow rate, the arm with the IV line should be positioned anterior to the patient rather than above the head. When an arm is extended above the head, the subclavian vein may be compressed under the clavicle with resultant obstruction of flow. Experience with evaluation of thoracic outlet compression shows that compression of venous structures is increased as the arm is extended further above the head.1 Because the arm cannot be left down at the patient’s side within the bore of the gantry, it should be positioned anterior to the patient in a vertical orientation where it can rest. To avoid artifact from electrocardiographic (ECG) leads, the two arm leads should be placed cephalad to the level of the heart; the left lower lead is placed in the region of the left midabdomen. Leads should be positioned in an area that is clean and free of hair to obtain good contact. An adequate ECG tracing with clearly defined R-waves must be observed before proceeding. When necessary, the position of the ECG leads should be adjusted to optimize the ECG tracing. A regular sinus rhythm is optimal for a gated cardiac examination. If the rhythm is irregular, the risks and benefits of the examination should be considered because an irregular rhythm is often associated with a suboptimal examination. When a coronary CTA examination is performed in a patient with an irregular rhythm, prospective ECG gating should not be used (see below). A baseline blood pressure should be obtained before the administration of β-blockers. Monitoring of blood pressure and heart rate should continue during and after administration of β-blockers to document stable vital signs before discharge. A low-dose, noncontrast calcium examination may be useful before coronary CTA is performed. The radiation exposure of a typical calcium study (about 1 mSv) is 10% of the dose associated with conventional helical coronary CTA.2,3 The presence of heavy coronary calcification, specifically an Agatston-130 calcium score above 1000, is associated with reduced image quality and diagnostic accuracy on coronary CTA (Figs. 2.1 and 2.2).4 When an elevated calcium score above 1000 is identified before coronary CTA, the option of proceeding directly to cardiac catheterization should be considered because CTA is unlikely to exclude the presence of stenosis. In patients who do proceed to coronary CTA, the calcium examination can be used to determine the proper superior and inferior endpoints for the coronary CTA study. The heart rate pattern during calcium scoring should also be evaluated because it may be predictive of the changes in rhythm that will be observed during coronary CTA. Calcium scoring is discussed in more detail in Chapter 5. In-plane spatial resolution varies within the axial plane and is highest near the center of the CT gantry. For this reason, it is important to center the scan on the heart. Most cardiac scans are performed with a field of view of 22 to 25 cm. The heart should be centered within this field of view in both the left–right and anterior–posterior axes. In our protocol, centering of the heart is performed when the bolus-tracking region of interest is defined (Fig. 2.3). A basic coronary CTA scan should begin at about 2 cm above the most superior point in the left anterior descending artery and extend 1 to 2 cm below the cardiac apex (Fig. 2.4). If a calcium scoring study is performed before coronary CTA, table positions for the CTA may be determined from the noncontrast calcium scoring images. If a calcium scoring study is not performed, coronary CTA should begin at the carina and extend 2 cm below the estimated lower margin of the heart on the topogram. When the ascending aorta is tortuous or the left heart border bulges superiorly, imaging should begin 1 to 2 cm above the carina to allow for a cranial angulation of the proximal left coronary artery (LCA). In a patient with venous bypass grafts or radial artery grafts from the ascending aorta, CTA begins at the top of the aortic arch. When evaluating a patient with internal mammary artery bypass grafts, the scan starts just above the level of the clavicles to include the origins of the internal mammary arteries. A “triple rule-out” scan usually begins just below the level of the clavicles to image the upper lobe pulmonary artery branches and the origins of the great vessels. The lower extent of the scan should extend just below the expected lower margin of the heart. In a patient with a gastroepiploic bypass graft, the scan should be extended further down into the abdomen to visualize the origin of this graft. Fig. 2.1 Mild coronary calcification with diagnostic-quality coronary CT angiography of the left anterior descending artery (LAD). (A) Maximum intensity projection (MIP) through the LAD demonstrates two areas of calcification, one at the origin of the vessel (arrow) and a second area more distally (arrowhead). The calcified plaque at the origin of the vessel obscures the entire lumen, and the more distal plaque results in approximately a 50% diameter narrowing. (B) Orthogonal MIP image through the LAD demonstrates that the proximal calcified plaque results in no more than 50% narrowing (arrow). In this projection, however, the more distal plaque appears to cover a larger portion of the vessel lumen. By combining the information from both images, one can conclude that there is no more than a 50% narrowing of the LAD. The absence of significant narrowing was confirmed at cardiac catheterization. Fig. 2.2 Extensive coronary calcification limits the value of coronary CT angiography. (A) Maximum intensity projection (MIP) image of the left anterior descending artery demonstrates diffuse calcification in the proximal portion of the vessel (large and small arrows). The calcified plaques appear to obstruct much of the lumen of the vessel. (B) Orthogonal MIP image demonstrates that the more proximal calcified plaque (large arrow) results in less than 50% narrowing; the more distal calcification (small arrow) overlies the entire width of the vessel. Coronary angiography demonstrated less than 50% narrowing. Dense calcification is often associated with blooming artifact that causes plaque to appear larger than its true size and results in overestimation of stenosis. Fig. 2.3 Locator image for tracking of the injection bolus during coronary angiography. (A) Axial locator through the level of the left atrium demonstrates a region of interest along the left side of the left atrium. The region of interest is placed posteriorly and to the left so that the examination is not inadvertently triggered by contrast in the right side of the heart. (B) Axial locator with region of interest in the left atrium. The coronary angiogram is triggered when the contrast bolus arrives in the left atrium. After the scan is triggered, this system requires 5 seconds to begin the actual CT angiogram series. This additional short delay provides adequate time for full arterial enhancement of the aorta and coronary circulation. Fig. 2.4 Topogram for planning of coronary CT angiography. (A) Anteroposterior scout demonstrates the cardiac silhouette with the planned levels for coronary angiography. The superior extent of the scan is just above the carina. The inferior extent of the scan is 2 cm below the cardiac apex. The inferior extent is positioned to provide adequate coverage of the coronary circulation in the event that the patient takes a deeper breath during the angiographic phase of the study. The locator image that is used to monitor enhancement during the injection is positioned below the carina at the level of the left atrium. (B) Planned levels for coronary angiography in a patient with a tortuous ascending aorta. The superior extent of the scan is positioned slightly higher because the left anterior descending artery may course superi-orly from its origin. The left-right centering places the heart in the center of the image. It is important to realize that patient dose is proportional to the length of the scan. Patient dose is minimized by limiting the scan to the levels necessary to visualize the coronary arteries. The rationale for imaging above the coronaries and below the heart is to ensure that the coronary arteries are visualized in their entirety in the event that the inspira-tory effort during the coronary CTA is different from the effort during the acquisition of the scout topogram or the calcium scoring study. Based on the Jefferson experience, the optimal starting and ending positions for coronary CTA include a 1–2 cm margin of error for respiratory variation. The ideal heart rate for this examination is regular sinus bradycardia in the range of 50 to 60 beats per minute. Regular sinus bradycardia is critical for single-source scanners with temporal resolution greater than 150 msec. Although this requirement may be relaxed for dual-source scanners with a temporal resolution of 75 to 83 msec and single-source scanners that acquire the entire coronary circulation in one or two cardiac cycles, a regular sinus bradycardia results in better image quality with all scanners. Even scans obtained with the most advanced equipment may be adversely impacted by a premature beat at an inopportune time during image acquisition. Coronary CTA may be performed without pre-medication in a minority of patients who present with a stable sinus bradycardia. However, it is important to determine whether the observed sinus bradycardia actually represents a stable rhythm. Anxiety may result in elevation of an initially favorable heart rate. Many patients will experience a sudden change in heart rate or rhythm while holding their breath or immediately after administration of IV contrast material. For the patient who presents in sinus bradycardia, stability of the heart rate should be confirmed on a continuous ECG tracing while speaking with the patient and practicing a breath-hold on the CT table before the study. Pre-medication should be administered to maintain stability of the rhythm for any patient who demonstrates heart rate variability before the study. Various techniques have been advocated for controlling patient heart rate during cardiac CT. The generally preferred method is to administer a β-blocker to the patient before performing the study. The most commonly used β-blocker for cardiac CT, metoprolol, may be administered orally or intravenously. Metoprolol has a half-life of about 4 hours after IV administration and is relatively cardioselective at low doses.5 The maximum effect of an orally administered dose might not be observed for an hour, whereas the maximum effect of an intravenously administered dose is apparent in 3 to 5 minutes. For patients who cannot tolerate prolonged β-blockade, a fast-acting β-blocker with a short half-life is an option. Esmolol is an ultrashort-acting cardioselective IV β-blocker with a half-life of approximately 9 minutes.6 Esmolol is administered as an IV bolus followed by an infusion. The effect of esmolol begins to wane almost immediately after the drug is discontinued. Unfortunately, maintaining a steady heart rate for cardiac CT with an esmolol infusion can be technically challenging because changes in the rate of infusion are associated with rapid changes in heart rate. For the typical patient, metoprolol is administered either orally or intravenously. Oral administration requires pre-treatment of the patient at least 1 hour before the examination. A 50-mg oral dose is well tolerated by most adults. If the reduction in heart rate is not sufficient with one dose, a second or third dose may be required. Because of variable absorption and sensitivity to this drug, it is difficult to predict a priori how long it will take to regulate the heart rate with oral metoprolol. To maintain a fixed patient schedule and to avoid long waiting times for our patients, IV administration of metoprolol is the preferred technique for control of heart rate at our institution. IV administration provides rapid regulation of heart rate that can be carefully monitored while the patient is placed on the CT table before the examination. Regulation of the heart rate with IV metoprolol is usually accomplished within 5 to 10 minutes while the patient is on the CT table. To save time, administration of the IV β-blocker is performed during acquisition of the topogram, calcium scoring images, and tracker images that are obtained before coronary CTA. Patients who arrive with a heart rate in the range of 60 to 65 beats per minute are given an initial IV dose of 2.5 mg of metoprolol. Patients who arrive with a heart rate above 65 beats per minute are given an initial IV dose of 5 mg. After allowing several minutes to observe the effect of the first dose, additional doses of 5 mg are administered every 3 to 5 minutes until the target heart rate is achieved. Blood pressure is monitored after every dose to maintain a systolic blood pressure greater than 100 mm Hg. The maximum dose of IV metoprolol in my practice is 30 mg, although it is rare to administer more than 20 mg. If the heart rate has not decreased substantially with the first 15 to 20 mg, it is unlikely that further dosing will be beneficial. When an oral β-blocker is given before coronary CTA, some centers prescribe 100 mg of metoprolol 1 hour before the examination. It is important to question the patient about any history of asthma, bradycardia, hypotension, or cocaine use before the β-blocker is prescribed. Ideally, a blood pressure should be obtained before metoprolol is prescribed. Although this dose is usually well tolerated, there is variation in patient sensitivity to the drug. Any patient who does not routinely take a β-blocker should be monitored after taking the oral dose because this medication can result in bradycardia, hypotension, and dizziness. Asthma represents a relative contraindication to β-blockade. In a patient with mild asthma, the referring physician should be consulted to decide whether the patient may tolerate 5 or 10 mg of IV metoprolol before the examination. A higher dose should not be administered because the cardioselective action of metoprolol may be lost at higher doses, with the potential to cause an acute asthmatic reaction. Administration of a β-blocker in a patient who has recently used cocaine may predispose to coronary vasospasm and should therefore be avoided. Calcium channel blockers may be considered as an alternative to β-blockers in an asthmatic patient or in the patient with atrial fibrillation. Diltiazem and verapamil suppress atrioventricular nodal function and, to a lesser extent, sinoatrial nodal function.7 Unlike β-blockers, calcium channel blockers do not induce bronchospasm; however, they are not as effective in slowing down sinus rhythm. Calcium channel blockers are more effective than β-blockers in slowing the ventricular rate of a patient in atrial fibrillation. If a calcium channel blocker is to be used for cardiac CT, diltiazem may be preferred because this drug demonstrates the least negative inotropic effect among the commonly used calcium channel blockers. Diltiazem may be given as an initial bolus of up to 0.25 mg/kg. Careful monitoring of blood pressure is advised when giving IV calcium channel blockers because these medications are potent vasodilators and tend to lower the blood pressure even more quickly than β-blockers. The combination of β-blockers with calcium channel blockers tends to cause an even more precipitous drop in blood pressure. The protocol for IV administration of β-blockers presented in this chapter is somewhat more aggressive than that suggested by the prevailing literature.8 Judicious IV administration of metoprolol and diltiazem with the patient in a supine position and with careful monitoring of the blood pressure is a safe procedure. Nonetheless, anyone administering IV β-blockers or calcium channel blockers should have training in cardiac resuscitation. A full discussion of the pharmacology of these drugs is beyond the scope of this chapter. Treatment of hypotension or bradycardia that results from administration of a β-blocker or calcium channel blocker should be titrated to the patient’s blood pressure and mental status. Basic treatment for hypotension includes elevating the patient’s legs and administering fluid to maintain blood pressure. Glucagon should be available for treatment of β-blocker overdose.9 Calcium gluconate or calcium chloride should be available for treatment of overdose with a calcium channel blocker.10 If these methods fail, more aggressive resuscitation with atropine and catecholamines may be required.11 Nitroglycerin is routinely administered to promote coronary vasodilatation during cardiac catheterization. Nitroglycerin spray is simple to administer and provides a metered dose of nitroglycerin that is rapidly absorbed. Sublingual nitroglycerin is also simple to administer. The vasodilatory effect on heart rate and blood pressure is usually evident within 2 minutes, with a maximum effect at 5 to 10 minutes. Administration of sublingual nitroglycerin before cardiac CT results in an average increase of 12 to 21% in the diameters of the proximal coronary arteries.12 In addition to increasing the diameter of coronary arteries, pre-treatment with sublingual nitroglycerin increases the number of visualized coronary side branches.13 Vasodilatation of the normal coronary artery will make the presence of nondistensible atherosclerotic plaque more obvious and can help to distinguish fixed lesions from arterial spasm.14 Finally, the use of nigroglycerin may improve the diagnostic accuracy of coronary CTA.15 It is important to realize that the combination of nitroglycerin with a β-blocker may precipitate hypotension on the CT table. Furthermore, administration of nitroglycerin is associated with a reflex tachycardia and transiently increased variability of the heart rate, which may actually degrade image quality.16 Using a conventional 64-slice CT scanner with a temporal resolution of 165 to 210 msec, the mild variation in heart rate caused by nitroglycerin can impact negatively on image quality. However, this variability of heart rate related to nitroglycerin is less of an issue in newer scanners with better temporal resolution. Although there is a paucity of controlled trials to demonstrate the benefit of sublingual nitroglycerin prior to coronary CTA, our experience suggests that nitroglycerin is beneficial to coronary image quality. For this reason, our protocol includes sublingual nitroglycerin (800 mcg), administered on the CT table 2 to 3 minutes before the scan, provided the heart rate is well controlled, blood pressure is adequate, and the patient is not currently taking a phosphodiesterase inhibitor for erectile dysfunction. The goal of contrast injection for coronary CTA is to achieve a steady, high level of arterial enhancement (ie, >300 HU) during the examination. For a dedicated cardiac study, the goal is to achieve a high level of enhancement in the left heart and coronary arteries. A biphasic injection technique is used to clear dense contrast out of the superior vena cava during imaging of the coronary vessels to reduce streak artifact (Fig. 2.5). For a triple rule-out study, the goal is to achieve sufficient contrast enhancement on both sides of the heart to allow simultaneous evaluation of the coronary and pulmonary arteries. Although some clinicians have described three-phase injection protocols, the two-phase techniques are simpler and generally adequate. Fig. 2.5 Five-chamber view during coronary CT angiography demonstrates intense contrast opacification of the left atrium (LA) and left ventricle (LV) as well as the aortic root (Ao). The right atrium (RA) and right ventricle (RV) have been cleared of contrast by a saline flush, providing clear visualization of the right coronary artery as it courses in the right atrioventricular groove. The saline flush is useful for coronary angiography, but it should not be used for a “triple rule-out” scan during which the pulmonary artery must also be opacified. The volume and rate to be used for contrast injection depend on the scan time. As a general rule, the contrast injection time should be approximately 3 seconds longer than the scan time. Using a 16-detector system with a scan time of about 20 to 25 seconds, the duration of the contrast injection should be at least 25 seconds (100 mL at 4 mL/sec), followed by a saline flush (40 mL at 4 mL/sec). Using a 40-detector system with a scan time of about 15 seconds, the duration of the contrast injection should be at least 18 seconds (100 mL at 5.5 mL/sec), followed by a saline flush (40 mL at 4 mL/sec). Using a 64-detector system with a scan time of about 10 seconds, the duration of the contrast injection should be 13 seconds (70 mL at 5.5 mL/sec), followed by a saline flush (40 mL at 4 mL/sec). Using a 256-detector system with a scan time of 5 to 6 seconds, the duration of the contrast injection should be 8 to 9 seconds (50 mL at 5.5 mL/sec), followed by a saline flush (40 mL at 4 mL/sec). When evaluating a post-bypass patient with a 64-detector system, the contrast injection rate is reduced to 5 mL/sec to compensate for an increased scan time of 12 to 13 seconds that is required to include the origins of the bypass grafts. When performing a triple rule-out study for coronary disease, dissection, and pulmonary embolism, a saline flush cannot be used because it would wash the contrast out of the pulmonary arteries. For these studies, the saline flush is replaced by a 1:1 dilution of contrast material with saline. Using a 64-detector system with a scan time of ~15 seconds, the duration of the contrast injection should be 14 seconds (70 mL at 5 mL/sec), followed by a second phase with dilute contrast (60 mL at 4 mL/sec). These injection protocols for helical coronary CTA are summarized in Table 2.1. A more detailed discussion of technique for triple rule-out studies is presented in Chapter 15. When imaging is performed with prospective ECG gating with a “step-and-shoot” technique, contrast volume may need to be adjusted to accommodate a slightly shorter or longer scan time, depending on the number of acquisition cycles (“steps”) required in the step and shoot protocol. The plateau level of enhancement obtained within the heart is related both to the rate of injection and to the iodine concentration used.17 To increase the level of arterial enhancement, one may increase either the rate of contrast injection or the iodine concentration in the contrast material. Based on pressure limitations, an injection rate above 5 to 6 mL/sec is often not possible. The maximum iodine concentration currently available for contrast material in the United States is 350 mg/mL. European studies have demonstrated better arterial enhancement with a concentration of 400 mg/mL.18,19
Technique, Protocols, Instrumentation, and Radiation Dose
 Patient Related Issues
Patient Related Issues
Patient Preparation
Intravenous Access
Patient Monitoring
 Planning the Scan
Planning the Scan
Calcium Scoring Preceding Coronary CT Angiography
Scan Position
 Optimizing Patient-Related Factors
Optimizing Patient-Related Factors
Heart Rate
Coronary Vasodilatation
 Optimizing Study Parameters
Optimizing Study Parameters
Contrast Injection
Scan Timing
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree