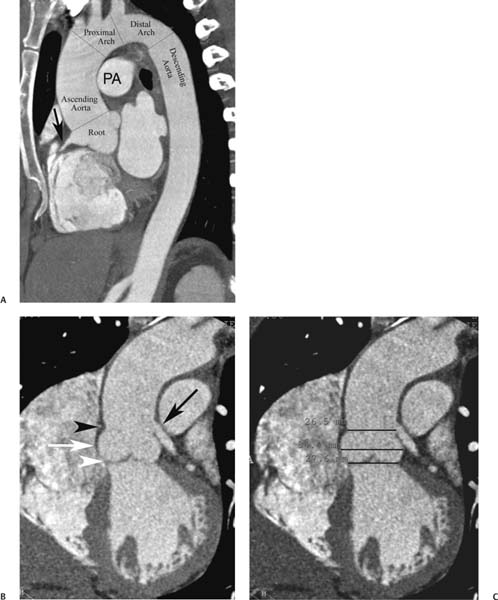
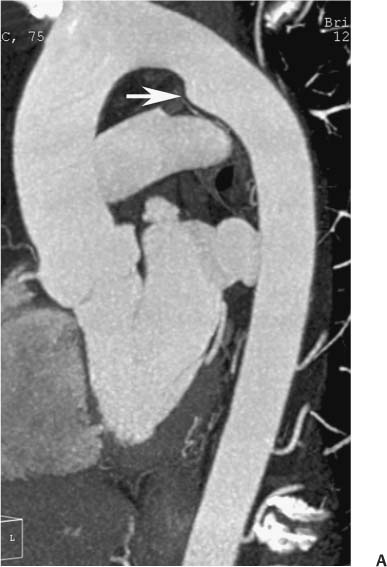
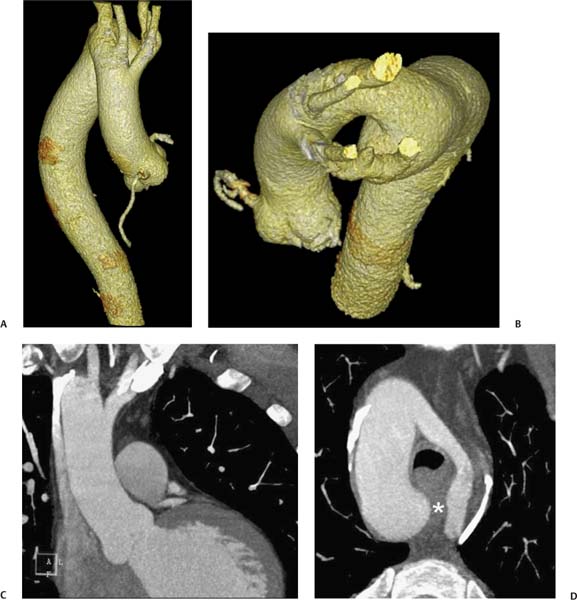
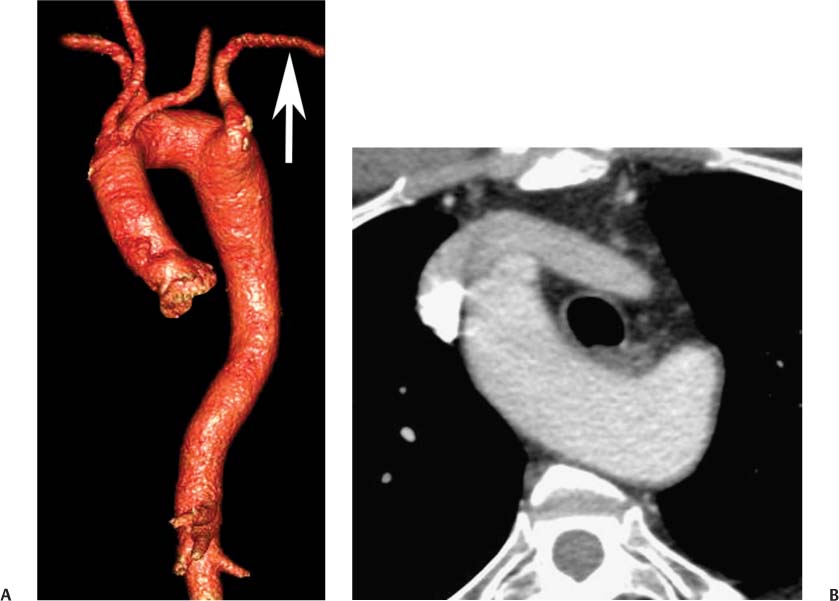
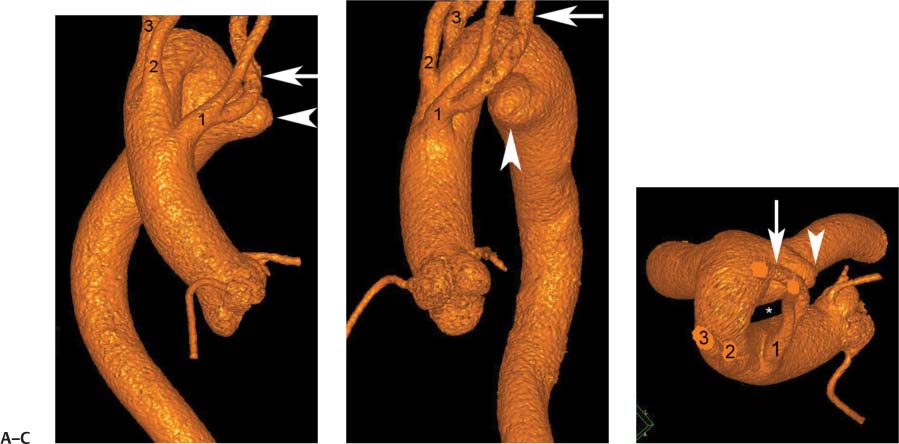
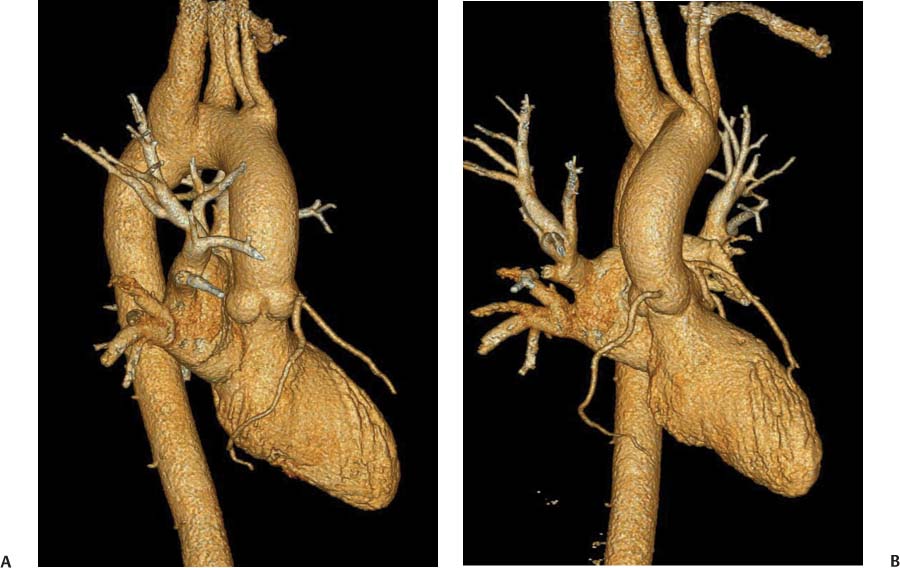
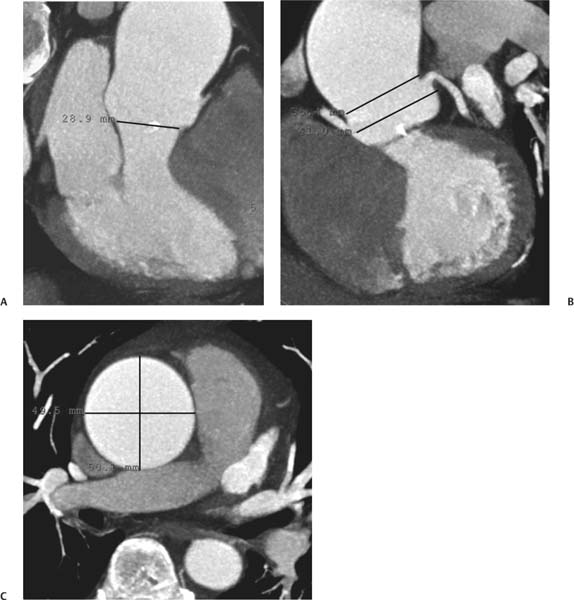
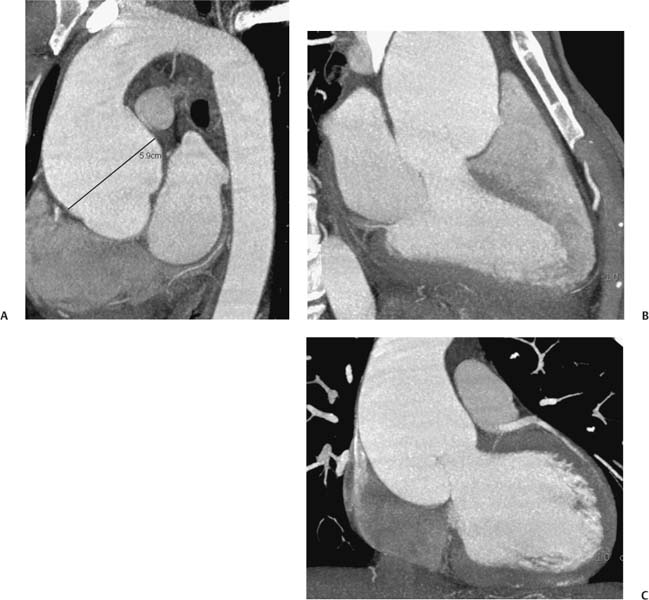
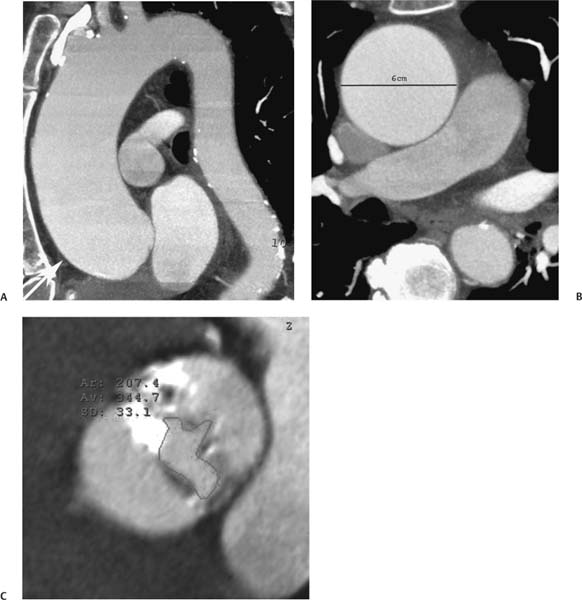
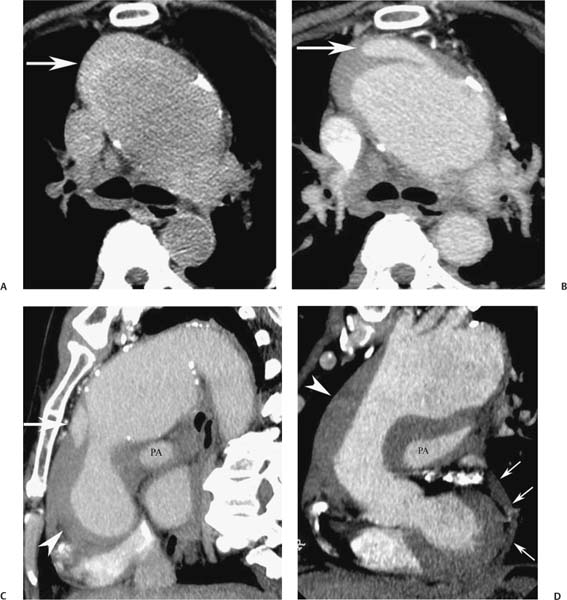
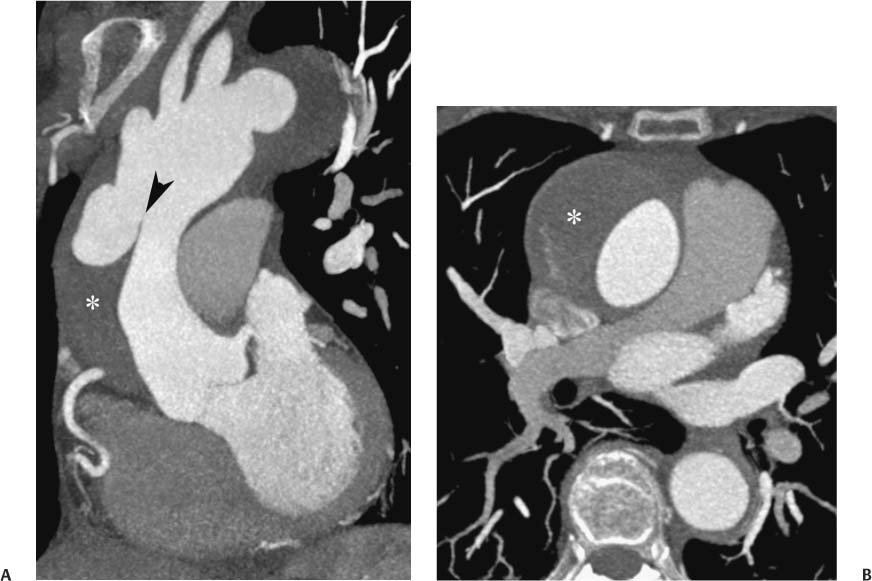
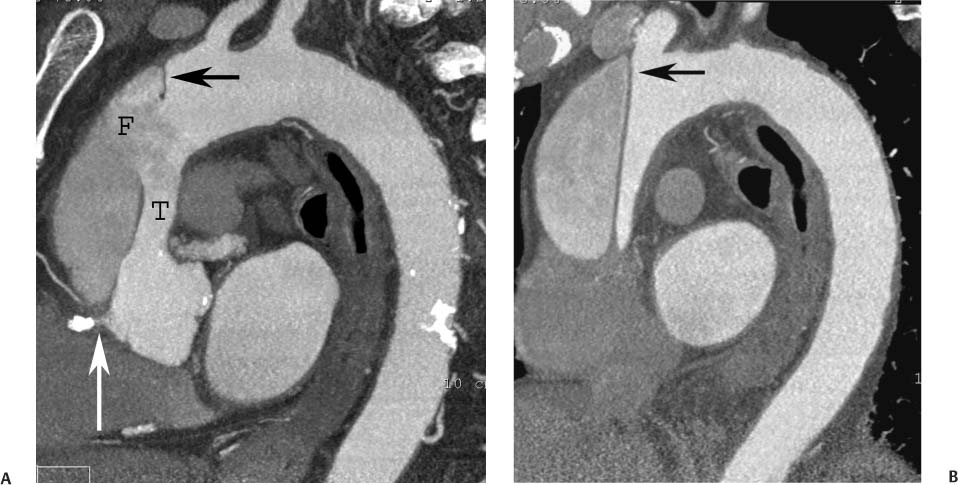
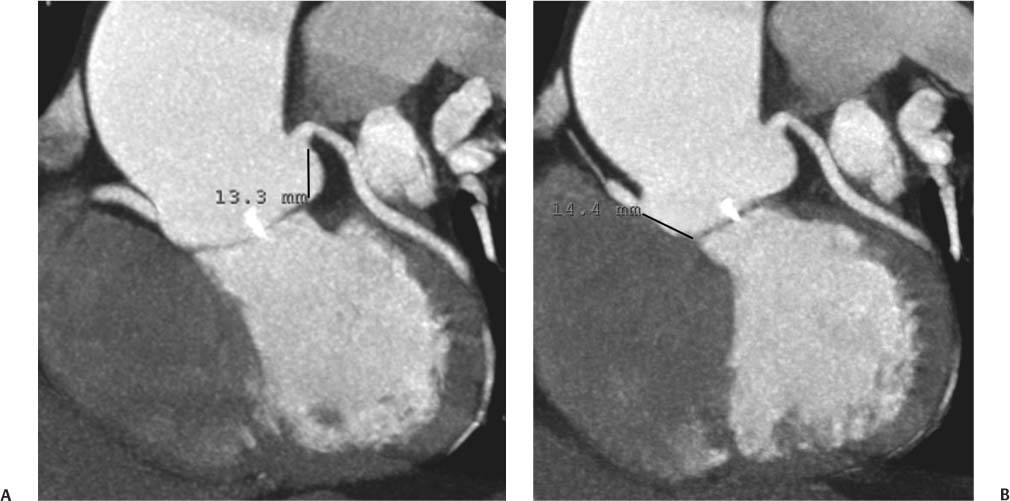
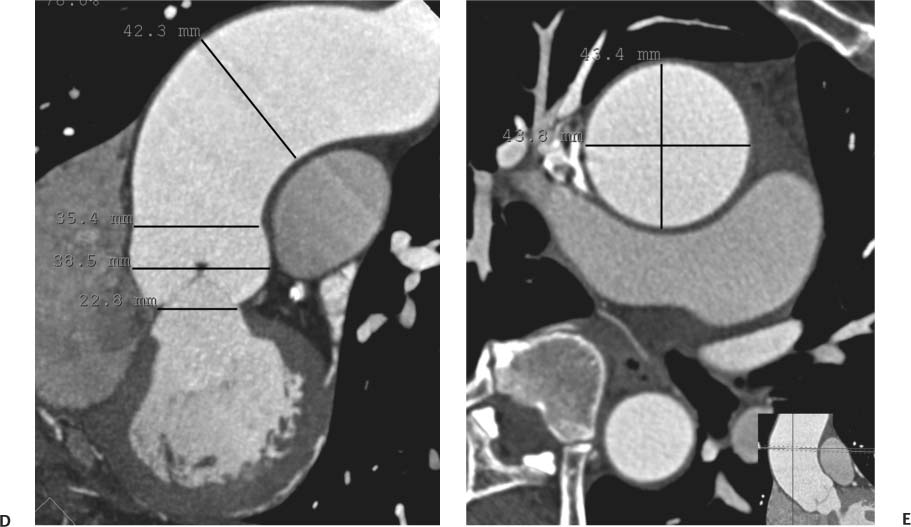
13 Assessment of the thoracic aorta is integral to evaluation of the cardiovascular system during cardiac CT. Changes in the structure and function of the thoracic aorta may significantly impact left ventricular function, coronary blood flow, and cerebral and peripheral circulation. Although dedicated coronary CT angiography (CTA) will not image the aortic arch, a complete interpretation of every cardiac CT should comment on the presence of thoracic aortic disease in visualized portions of the aorta. For patients in whom more complete evaluation of the aorta is desired, the electrocardiogrphic (ECG)-gated examination is easily extended from the aortic arch down through the diaphragm. Aortic anomalies, variants, aneurysmal disease, dissection, intramural hematoma, and penetrating atherosclerotic ulcers are clearly defined by ECG-gated CTA. This information allows the clinician to evaluate thoroughly aortic disease, stratify risk and prognosis, target medical therapy, plan endovascular and surgical interventions, and assess follow-up. Changes on serial assessment of the thoracic aorta can identify patients at increased risk for adverse outcome. Patients with rapid progression of disease over time may warrant more aggressive risk factor modification; blood pressure control; or early, timely surgical intervention. Finally, follow-up imaging after surgical intervention on the heart and thoracic aorta is essential for the early detection and treatment of complications and progression of disease. The thoracic aorta is divided into five segments: aortic root, ascending aorta, proximal aortic arch, distal aortic arch, and descending thoracic aorta (Fig. 13.1A). The aortic root is the cylindrical segment of aorta from the ventriculoaortic junction to the sinotubular junction, which contains the aortic valve, the aortic annulus, and the sinuses of Valsalva (see Fig. 3.1). The three aortic root sinuses or sinuses of Valsalva are functionally identical. The left and right coronary arteries normally arise from ostia in the left and right sinuses, respectively. The third, posterior, sinus is normally without a coronary origin and is termed the noncoronary sinus. The semilunar attachments of the aortic valve cusps form the hemodynamic junction between the left ventricle and the aorta. Structures distal to this boundary are exposed to arterial pressure; structures proximal to this boundary are subject to ventricular pressure. This relationship becomes more important in interpreting anatomic and physiologic changes in diseases affecting the aortic root. The attachments of the aortic valve cusps to the aortic wall are not planar, but rather crown-like, from the top of the commissures at the level of the sinotubular junction to the nadir of the cusps in the left ventricular outflow tract.1 Because of this anatomy, significant dilation of the aorta at the sinotubular junction leads to loss of valvular support with aortic valve incompetence.1,2 Understanding this mechanism of aortic insufficiency is essential to understanding changes in the aortic valve from diseases such as aortic dissection and annuloaortic ectasia. Aortic root size measurements are made at the annulus (nadir of the aortic leaflets), at the midpoint of the sinuses of Valsalva, and at the sinotubular junction (Figs. 13.1C,D). Assessing these dimensions allows accurate serial comparison of changes in aortic root size and defines the root morphology to allow disease classification and to plan surgical intervention.1,3,4 The size of the normal aortic root is related to body size, height, and sex. Aortic root size changes with age and with hypertension.4–6 Enlargement of the aortic root may lead to significant aortic valvular regurgitation in patients with hypertension, collagen vascular diseases (such as Marfan syndrome or Ehler-Danlos syndrome), and aortic dissection through loss of valve support and central aortic insufficiency. The normal diameter of the adult aorta just above the sinotubular junction averages 3.6 cm (range, 2.4–4.7 cm).7,8 The ascending aorta extends from the sinotubular junction to the origin of the innominate artery. The average diameter of the adult ascending aorta is 3.5 cm (range, 2.2–4.7 cm).8 A diameter greater than 4.0 cm is generally classified as aneurysmal (Figs. 13.1D,E). The aortic arch begins at the origin of the right innominate artery and ends at the attachment of the ligamentum arteriosum. The proximal arch extends from the origin of the right innominate artery to the origin of the left subclavian artery and includes the origin of the left common carotid artery. The distal arch, called the aortic isthmus, includes the aorta from the origin of the left subclavian artery to the ligamentum arteriosum. The distal aortic arch may be slightly narrower than the proximal descending thoracic aorta, particularly in infants and children. The descending thoracic aorta begins distal to the ligamentum arteriosum and extends to the aortic hiatus of the diaphragm. Its proximal portion may appear slightly dilated and is thus termed the aortic spindle. The mid-descending thoracic aorta has an average diameter of 2.5 cm (range, 1.6–3.7 cm). The distal descending thoracic aorta above the diaphragm has an average diameter of 2.4 cm (range, 1.4–3.3 cm).7,8 Fig. 13.1 Segments of the thoracic aorta. (A) The aortic root is visualized with three sinuses of Valsalva and the origin of the right coronary artery from the anterior sinus (arrow). The ascending aorta extends from the sinotubular junction to the origin of the innominate artery. The arch is divided into a proximal segment that extends to the origin of the left subclavian artery and a distal segment that extends to the ligamentum arteriosum. The mild dilatation of the descending aorta beyond the aortic isthmus is known as the aortic spindle. PA, pulmonary artery. (B) The normal proximal aorta demonstrates a tapered waist at the sinotubular junction (black arrowhead). Measurements of the proximal aorta include the diameter of the annulus at the level of the aortic valve (white arrowhead), the diameter of the root at the sinuses of Valsalva (white arrow), and the diameter of the aorta at the sinotubular junction (black arrowhead). Although the coronary arteries normally originate just below the sinotubular junction, the origin of the left coronary artery in this patient (black arrow) is just superior to the sinotubular junction. (C) Measurements of the aortic root are demonstrated at the aortic annulus (27.6 mm), the aortic root (34.4 mm), and the sinotubular junction (26.5 mm). Segments of the thoracic aorta. (D) Measurements of the aortic root and ascending aorta in a different patient demonstrate a top normal-size root (38.5 mm) and a mildly dilated ascending aorta (42.3 mm). A tapered diameter is measured at the sinotubular junction (35.4 mm). (E) Short-axis measurement of the ascending aorta in the same patient as (D) demonstrates aneurismal dilatation to 4.3 × 4.4 cm. Inset in the bottom right corner demonstrates angulation used to obtain short-axis measurement. The unique properties and anatomic relationships of the ligamentum arteriosum may create radiographic appearances that mimic thoracic aortic disease states. The ductus diverticulum is a focal, convex bulge on the undersurface of the isthmic region of the aortic arch (Fig. 13.2). This bulge is commonly thought to arise from the remnant of the ductus arteriosum, although some have theorized its presence as a vestige of the embryonic right dorsal aortic root.9 The importance of this structure is differentiating its appearance from the presence of a post-traumatic or atherosclerotic pseudo-aneurysm in this location (Figs. 13.19 and 13.20). The ductus diverticulum may be differentiated from an aortic pseudo-aneurysm by its smooth margins and symmetric appearance. Pseudocoarctation of the aorta may occur when the aorta elongates and kinks in this location resulting from tethering of the aorta at the ligamentum arteriosum. The appearance of the aorta in pseudocoarctation is similar to true coarctation, but the absence of collateral circulation readily differentiates the two states. Three sequential arterial branches arise from the aortic arch. The right innominate (brachiocephalic) artery arises first and is the largest, giving rise to the right subclavian and right common carotid arteries. The left common carotid artery arises next and is typically the smallest artery of the great vessels. The left subclavian artery branches next, arising from the distal arch posteriorly. The standard branching pattern is seen overall in about 70% of individuals.10 The most common variation is a combined origin of the innomi-nate and left common carotid arteries in about 10 to 13% of individuals, often termed a bovine aortic arch. The term bovine aortic arch is actually a misnomer because the branching pattern in cattle has a single trunk that splits into all the great vessels. In about 5% of the population, the left vertebral artery arises as a separate branch directly from the aorta, between the left common carotid artery and the left subclavian artery.10 Fig. 13.2 Ductus diverticulum in two patients. (A) Ductus diverticulum presents as a focal, convex bulge on the anterior undersurface of the isthmic region of the aortic arch (arrow). The smooth contour of the ductus diverticulum distinguishes this structure from a pseudoaneurysm. (B) Ductus diverticulum (arrowhead) associated with a patent ductus arteriosus (arrow) between the aorta (Ao) and pulmonary artery (PA). Congenital anomalies of the aorta and branching pattern of the great vessels may present as isolated anomalies or in association with other congenital cardiac malformations. Definition of the aortic malformation is based on the position and caliber of the aortic arch and its branches, the descending aorta, and the ductus or ligamentum arteriosum. Vascular rings are unusual congenital abnormalities in which the anomalous configuration of the arch or associated vessels forms a complete ring surrounding the trachea and esophagus and may result in symptoms related to compression. Vascular rings result from malformations of the primarily paired aortic arch or branching pulmonary arteries during embryogenesis. The two most common types of complete vascular rings are double aortic arch (Fig. 13.3) and right aortic arch with an aberrant left subclavian artery (Fig. 13.4), comprising 85 to 95% of vascular rings. Double aortic arch results from a persistence of the embryologic double aortic arch with right and left arches arising from the ascending aorta and rejoining posteriorly after giving rise to their respective carotid and subclavian arteries. In the right aortic arch with an aberrant left subclavian artery, a left ligamentum arteriosum may connect the aberrant subclavian artery to the left pulmonary artery to complete the ring. Two other complete vascular rings that are extremely rare (<1%) include the right aortic arch with mirror-image branching and a left ligamentum arteriosum (Fig. 13.5) and left aortic arch with an aberrant retroesophageal right subclavian artery (Fig. 13.6), right-sided descending aorta, and right ligamentum arteriosum.11 In infants and children, compression of the trachea and esophagus from vascular rings frequently cause symptoms of respiratory distress, recurrent pneumonia, bronchitis, stridor, and poor intake.12 Related vascular anomalies involving arch vessels that do not form a complete ring may produce similar symptoms from compression of the trachea or esophagus or may remain asymptomatic.12 A right aortic arch crosses the mediastinum to the right of the trachea and esophagus and occurs in 0.1% of adults.13,14 Right aortic arch anomalies include right aortic arch with an aberrant left subclavian artery; right aortic arch with mirror image branching; and right aortic arch with isolated left subclavian artery arising from the left pulmonary artery. Of these, only the right aortic arch with aberrant left subclavian artery is likely to be a source of symptomatic tracheal–esophageal compression. Right aortic arch with mirror-image branching has a high likelihood of association with other forms of complex congenital heart disease but is not usually itself a cause of symptoms.14 Isolated left subclavian artery is a rare cause of upper extremity ischemia. Fig. 13.3 Double aortic arch forms a vascular ring around the trachea and esophagus. (A) Anterior view demonstrates a larger right arch and a smaller left arch, each giving rise to an ipsilateral carotid and subclavian artery. (B) View from above clearly demonstrates the ring created by the two arches. (C) Coronal maximum intensity projection image demonstrates the ascending aorta as it divides into a larger right arch and a smaller left arch. (D) Axial view demonstrates that the trachea and esophagus are encircled by the vascular ring. The esophagus (*) is compressed from both sides. Vascular rings are rare in adults. In one series of vascular rings first diagnosed in adults, the most common anomaly was double aortic arch (46%) (Fig. 13.3), followed by right aortic arch with aberrant left subclavian artery and ligamentum arteriosum (30%) (Fig. 13.4). Only two thirds of adults in this report were symptomatic at diagnosis. Dysphagia and respiratory symptoms were the predominant presenting symptom in adults.15–17 Exercise-induced dilatation of the aortic arch and age-dependent changes in thoracic compliance have been proposed as potential mechanisms of new-onset symptoms in the adult-diagnosed vascular ring patient.15,16 Symptomatic vascular rings require operative intervention. Fig. 13.4 Right-sided aortic arch with aberrant left subclavian artery. (A) Left anterior oblique view demonstrates four vessels arising from the right-sided arch. In the order of their origins from the arch, these vessels are the left common carotid artery, the right common carotid artery, the right subclavian artery, and the left subclavian artery (arrow). Note that the left subclavian artery originates with a broad-based origin—the diverticulum of Kommerell—from the distal aortic arch. (B) Axial image demonstrates the right-sided arch with the diverticulum of Kommerell passing behind the trachea and esophagus and giving rise to the aberrant left subclavian artery. A vascular ring may be completed by a left-sided ductus arteriosus (or its remnant, the ligamentum arteriosum) passing from the aberrant left subclavian artery to the proximal left pulmonary artery. CTA is the ideal imaging modality to assess vascular rings. Three-dimensional reconstruction with and without volume rendering defines the morphology of the great vessels and their topography in relation to the adjacent soft tissue structures and allows pre-operative planning for surgical intervention. CT offers significant advantage over echocardiography in the delineation of the great vessels to the adjacent esophagus, trachea, and bronchi for surgical planning as well.11 Aneurysms of the thoracic aorta are most common in the ascending portion (Fig. 13.7). Whereas atherosclerosis is a common cause of all thoracic aortic aneurysms, this cause is rare for isolated ascending aortic aneurysms. The mechanism for isolated ascending aortic aneurysms is likely based on a genetic or acquired weakness in the aortic wall that renders these patients susceptible to hemodynamic-induced enlargement with time. Cystic medial degeneration is the most common cause of isolated ascending aortic aneurysm disease. In many patients, cystic medial degeneration may be the result of susceptibilities from genetic disorders such as Marfan syndrome, Ehler-Danlos syndrome, familial aneurysm disease, bicuspid aortic valve disease, or the inflammatory processes of infectious or noninfectious aortitis, including syphilis and Takayasu arteritis. Up to one third of cases are idiopathic with acceleration of the normal aging processes of elastic fiber fragmentation and smooth cell necrosis for unknown reasons.18 Idiopathic cystic medial degeneration may represent an undefined susceptibility for accelerated degeneration in response to risk factors such as smoking and hypertension. Marfan syndrome, a common inherited connective tissue disease caused by deficiency of the matrix protein fibrillin-1, affects the ocular, skeletal, and cardiovascular system.19,20 Patients with Marfan syndrome demonstrate accelerated aneurysm growth and tend to dissect and rupture at smaller sizes, especially in patients with a family history of early complications and death. The average age of death for untreated patients with Marfan syndrome is 32 years, with aortic root complications implicated in 60 to 80% of deaths from acute heart failure, acute aortic dissection, and aortic rupture.21 The classic aneurysm features in Marfan syndrome include a pear-shaped aneurysmal ascending aorta with smooth tapering to a normal aortic arch and has been termed annuloaortic ectasia. This condition is characterized by dilated sinuses of Valsalva with effacement of the sinotubular junction (Fig. 13.8). Fig. 13.5 Right-sided aortic arch with mirror image branching in an adult patient with dysphagia lusoria presenting as chest pain. (A) AP view demonstrates a right sided aortic arch with three great vessels. The first branch is a left innominate artery (1), followed by a right common carotid artery (2), and finally a right subclavian artery (3). The descending aorta is on the right side. A remnant of the distal portion of the left aortic arch presents as a diverticulum of the descending aorta (arrowhead), just posterior to the left subclavian artery (arrow). (B) Steep left anterior oblique projection demonstrates the proximity of the left subclavian artery (arrow) to the aortic diverticulum (arrowhead). In this highly unusual case, a fibrous connection between these two structures results in a vascular ring with associated esophageal compression. More commonly, a vascular ring may be present in a right-sided arch when a left-sided ductus arteriosus or ligamentum arteriosum passes between the left-sided descending aorta and the proximal left pulmonary artery. (C) View from above demonstrates the ring formed by the right arch, the left subclavian artery (arrow) and its fibrous connection to the aortic diver-ticulum (arrowhead). The trachea and esophagus pass within the ring (*). Fig. 13.6 Left-sided aortic arch with aberrant right subclavian artery. (A) Anterior view demonstrates the left-sided arch and left-sided heart structures, including the pulmonary veins. An aberrant subclavian artery originates from a broad-based diverticulum of Kommerell in the distal aortic arch. (B) Left anterior oblique view demonstrates the right coronary artery originating from the anterior sinus of Valsalva. The large aberrant subclavian artery is the fourth and final branch of the arch. (C) Posterior view and (D) coronal maximum intensity projection demonstrate the aberrant origin of the right subclavian artery with a broad base from the distal aortic arch. The descending aorta remains on the left side. (E) Oblique axial view demonstrates the aortic arch to the left of the trachea and the origin of the aberrant right subclavian artery posterior to the trachea. A vascular ring is present when a right-sided ductus arteriosus or ligamentum arteriosum passes from the aberrant subclavian artery to the right pulmonary artery. Bicuspid aortic valve disease is the most common cardiac congenital anomaly, occurring in up to 2.0% of the population in the United States. Bicuspid aortic valve patients have an intrinsic smooth muscle abnormality that predisposes them to higher rates of cystic medial degeneration and ascending aortic enlargement and dissection.22 These patients have an increased incidence of both aortic root enlargement and ascending aortic aneurysms independent of the function of their bicuspid valves, age, body size, and hypertension.6,22 Patients with bicuspid aortic valve disease and aneurysmal dilation of the ascending aorta should be considered for earlier surgery because of the intrinsic weakness of the aorta (Fig. 13.9). Fig. 13.7 Aneurysm of the aortic root and ascending aorta with preservation of the sinotubular junction. (A) Three-chamber view demonstrates measurement of the aortic annulus (2.9 cm) at the level of a mildly calcified aortic valve. (B) Left anterior oblique view demonstrates measurement of the aortic root at the level of the sinuses of Valsalva (4.1 cm) and at the sinotubular junction (3.5 cm). Although the root is dilated, a normal tapered appearance is present at the sinotubular junction. (C) Axial image through the ascending aorta demonstrates an aneurysm measuring 5 cm in diameter. Syphilitic aortitis causes destruction of the aortic media with loss of elastic and smooth muscle fibers and subintimal scarring. These changes lead to aortic dilatation and aneurysms. The most common site of a syphilitic thoracic aneurysm is the ascending thoracic aorta, followed by the aortic arch, proximal descending thoracic aorta, and distal descending thoracic aorta. Sinus of Valsalva involvement is rare and usually asymmetric. Despite infrequent sinus involvement, syphilitic aortitis may be associated with narrowing of the coronary ostia due to subintimal scarring with myocardial ischemia.23 The goal of surgical intervention on asymptomatic patients with aortic dilatation is to prevent rupture (Fig. 13.10) and aortic dissection (Figs. 13.11 and 13.12). In persons younger than 60 years, an ascending aortic diameter greater than 4 cm and a descending aortic diameter greater than 3 cm indicates dilatation; a diameter greater than 1.5 times the expected normal diameter is defined as a thoracic aortic aneurysm.24 The diameter of an aneurysm strongly correlates with the risk of rupture or dissection, but there is not a clear consensus of an absolute size indication for elective surgical repair in ascending aortic aneurysms.20 Fig. 13.8 Annuloaortic ectasia with effacement of the sinotubular junction. (A) Left anterior oblique view demonstrates the “candy cane” view of the thoracic aorta with dilatation of the aortic root and proximal ascending aorta to 5.9 cm. (B) Three-chamber view is approximately perpendicular to the view in (A) and again demonstrates dilatation of the proximal aorta with effacement of the sinotubular junction. (C) Coronal view demonstrates a dilated aorta above the aortic valve with no hint of a sinotubular junction. The Yale group reported rupture or dissection at a median size of 5.9 cm in the ascending aorta and 7.2 cm in the descending aorta with yearly rates of rupture or dissection about 7% and death 12% at or above these sizes.19,20 On the basis of these data, the traditional threshold for elective surgical therapy in good-risk candidates has been 5.5 cm for ascending aortic aneurysms. To improve risk stratification, some centers have advocated using cross-sectional area with indexing to body size. Although volume measurements have been advanced by some as the best estimate of the risk of rupture, there are no adequate natural history data for this approach.24 Others have suggested the use of ratios of measured to expected size based on the body surface area and age, adjusted according to the underlying etiology.25 The rate of expansion is also an important consideration in thoracic aneurysm disease. In a large longitudinal study, the average rate of growth for all thoracic aneurysms was found to be 0.12 cm per year.19 Longitudinal studies of the rate of expansion have reported greater rates of expansion in the descending aorta with Marfan syndrome, chronic dissection, and bicuspid valve disease.26 Growth at a rate of greater than 1.0 cm per year is a widely accepted indication for surgical intervention.25 Fig. 13.9 Aortic aneurysm with bicuspid aortic valve. (A) Left anterior oblique view demonstrates a diffusely dilated ascending aorta (arrow) with no evidence of normal tapering at the sinotubular junction. (B) Axial image demonstrates a maximum diameter of 6 cm in the ascending aorta. (C) Systolic phase image through the aortic valve demonstrates a calcified bicuspid aortic valve with mild stenosis (valve area = 2.1 cm2). A bicuspid aortic valve is associated with abnormal properties of the ascending aorta that predispose to aortic aneurysm, dissection, and rupture at smaller diameters than the general population. The individual benefit–risk assessment for intervention is based on the patient’s composite risk of rupture and dissection as a function of size, rate of growth, etiology, associated disease, and surgical risk. Size thresholds for intervention in individual patients are adjusted for underlying etiology, recent rate of growth, and family history of complications. Patients with Marfan syndrome, Ehler-Danlos syndrome, familial aneurysms, and chronic dissection all likely benefit from earlier intervention. Patients with bicuspid aortic valves may be at increased risk and may warrant early surgical treatment.6,27 Preoperative CTA of the aorta is essential for surgical planning. Accurate assessment of aortic root size, rate of change, morphology of the aortic valve leaflets, and distal extent of the ascending aortic aneurysm is critical to plan the appropriate operation. Further presurgical assessment includes the presence of concomitant coronary disease, aortic valvular stenosis, and regurgitation and the status of the mitral valve. In patients with large aneurysms and effacement of the sinotubular junction, measurements of the aortic root diameter may be misleading. In these cases, assessing the cephalad displacement of the coronary ostia provides a measure of the integrity of the sinuses of Valsalva and the aortic root (Fig. 13.13). Assessing whether aortic regurgitation is related to the valve itself or to loss of sinotubular support (as seen in annuloaortic ectasia) is important when considering root preservation and valve-sparing techniques. Fig. 13.10 Ascending aortic aneurysm with contained rupture into the pericardial space. (A) Axial view without intravenous contrast demonstrates a dense rim of hematoma (arrow) around the ascending aorta. (B) Post-contrast view at the same level demonstrates an aneurismal ascending aorta with contrast extending beyond the aorta anteriorly (arrow) into the hematoma. (C) Sagittal view again demonstrates contrast (arrow) anterior to the true lumen of the ascending aorta with hematoma extending down to the aortic root (arrowhead) and surrounding the pulmonary artery (PA). (D) Oblique view demonstrates hematoma anterior to the ascending aorta (arrowhead), surrounding the PA, and extending caudally inside the pericardium. Ascending aortic aneurysm with contained rupture into the pericardial space. (E) Pre-contrast axial image demonstrates dense hematoma (arrowheads) in the pericardial space around the heart. (F) Pericardial fluid appears lower in density on a contrast-enhanced image at the same level (arrowheads). These findings had progressed from a study performed earlier the same day. The patient was taken to surgery, which demonstrated rupture of a thoracic aortic aneurysm into the pericardial space. Ascending aortic aneurysms with normal sinuses and a normal aortic annulus may be treated with replacement of the ascending aorta from the sinotubular ridge to the origin of the innominate artery using a simple tube graft. If the aortic valve is diseased, a standard aortic valve replacement may be performed. In patients with marked effacement of the sinotubular junction and cephalad displacement of the coronary ostia, the aortic root should be replaced as a part of the index procedure. Patients with Marfan syndrome with significant dilatation of the aortic root and ascending aortic aneurysm should undergo replacement of both the aorta and root because of the high frequency of subsequent root-related complications and death. This approach involves replacing the ascending aorta and root with a valve conduit, and reimplantation of the coronary arteries into the neosinuses. Fig. 13.11 Type A dissection with aneurysm of the ascending aorta (DeBakey type I). (A) Left anterior oblique projection of the ascending aorta demonstrates a dissection flap just proximal to the arch vessels (arrowhead). Extensive thrombus is present in the false lumen (*), extending down to the aortic root. (B) Axial view through the ascending aorta demonstrates aneurismal dilatation of the aorta with compression of the true lumen of the aorta by a thrombus-filled false lumen (*). (C) Axial image through the arch demonstrates a more distal dissection flap (arrowhead) with thrombus in the false lumen along the arch (*). Any extension of this dissection may threaten the integrity of the aortic root, the coronary arteries, or the great vessels. Involvement of the sinuses of Valsalva in non-Marfan syndrome patients may be treated with valve-sparing aortic root reconstruction techniques or replacement of the aortic valve and reimplantation of the coronary arteries. In patients with extensive disease involving the arch or descending thoracic aorta, additional procedures such as arch replacement and “elephant trunk” procedures may be performed under the same deep hypothermic circulatory arrest to treat all diseased aortic tissue and plan for additional future procedures. Novel approaches to treating aortic arch and descending thoracic aortic disease simultaneously through combined open surgery and endovascular stent graft deployment are currently under development.28 Fig. 13.12 Type A dissection of an aneurysmal ascending aorta (DeBakey Type II). (A) Left anterior oblique view demonstrates that the proximal extent of the dissection flap is at the ostium of the stented right coronary artery (white arrow). The distal extent of the flap is in the proximal arch (black arrow). There is bidirectional flow of blood between the true lumen (T) and false lumen (F) though a rent in the intimal flap. (B) Slightly different obliquity demonstrates that the distal extent of the dissection flap ends at the origin of the innominate artery (arrow). Dissection into a coronary artery or into a great vessel is an important source of morbidity and mortality with type A dissection. Type A dissection of an aneurysmal ascending aorta (DeBakey Type II). (C) Axial image demonstrates the intimal flap (arrows) with a brightly enhanced true lumen and a larger false lumen. The diameter of the ascending aorta at the level of the dissection (5.6 cm) measures approximately twice the diameter of the descending aorta. All patients who have undergone thoracic aortic surgery should have long-term follow-up with repeat imaging. Because complete resection of aortic tissue is not feasible and residual aortic tissue is often not normal, patients are at risk for the subsequent aortic dissection, aneurysmal degeneration, and pseudoaneurysm formation. Routine scheduled surveillance CT or magnetic resonance imaging (MRI) is ideal for monitoring disease progression and for early detection of complications to avoid the increased morbidity and mortality of emergency reoperation. Patients at increased risk of reoperation, such as those with Marfan syndrome, familial aneurysms, or dissections, require more frequent follow-up. Fig. 13.13 Evaluation of the aortic root with measurement of the cephalad displacement of the coronary ostia from the aortic annulus. (A) Measurement of the superior displacement of the ostium of the left main coronary artery (in the same patient as figure 13.7). (B) Measurement of the superior displacement of the ostium of the right coronary artery. The coronary ostia are located just below the sinotubular junction (in the same patient as Fig. 13.7). (C) Three chamber view in a different patient demonstrates dilatation of the aortic root to 4.3cm. (D) Measurements of the superior displacement of the coronary ostia in this second patient demonstrate that most of the dilatation is related to the right sinus of Valsalva. The RCA origin is 2.8cm above the aortic annulus, while the LCA origin is 2.0cm above the aortic annulus. The superior displacement of the coronary ostia provides the surgeon with a measurement of expansion and deformation of the aortic root.
Thoracic Aorta
 Normal Anatomy of the Aorta
Normal Anatomy of the Aorta
 Thoracic Aorta: Normal Variants
Thoracic Aorta: Normal Variants
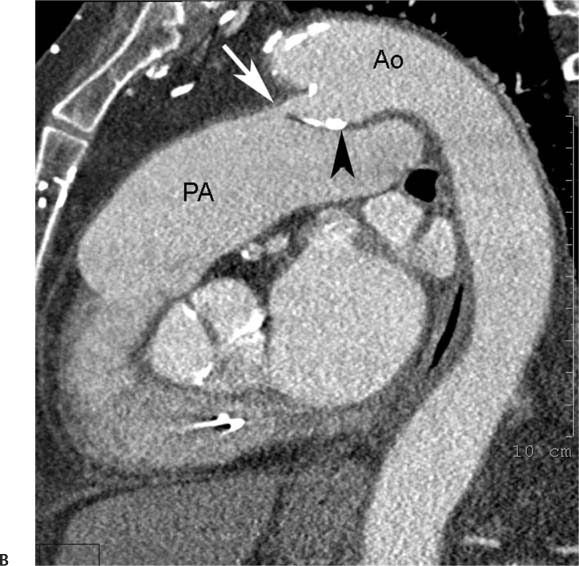
 Congenital Anomalies of the Aorta and the Great Vessels
Congenital Anomalies of the Aorta and the Great Vessels
 Aneurysmal Disease of the Ascending Aorta
Aneurysmal Disease of the Ascending Aorta
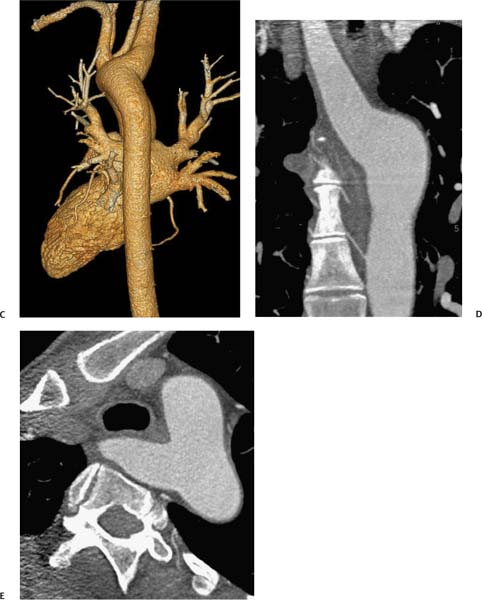
Aneurysm Size
Growth
The Influence of Etiology
Surgical Planning
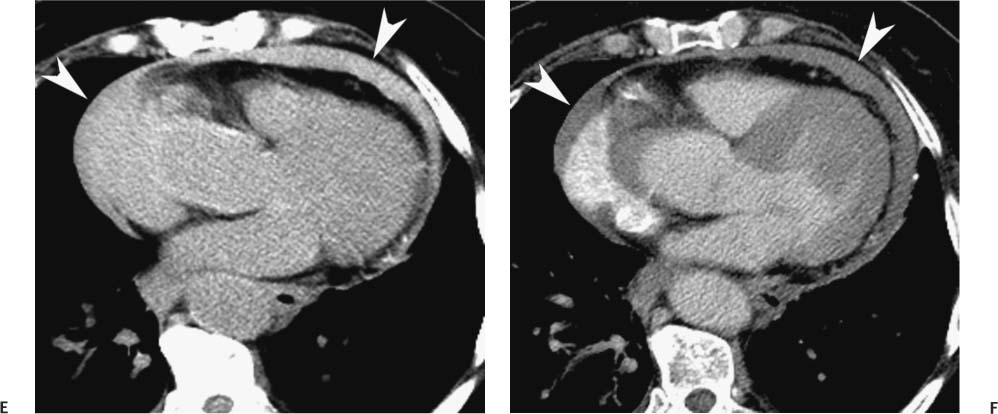
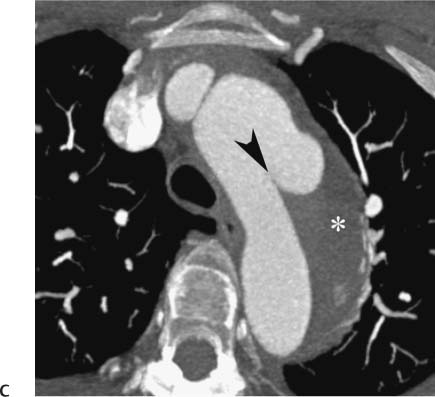
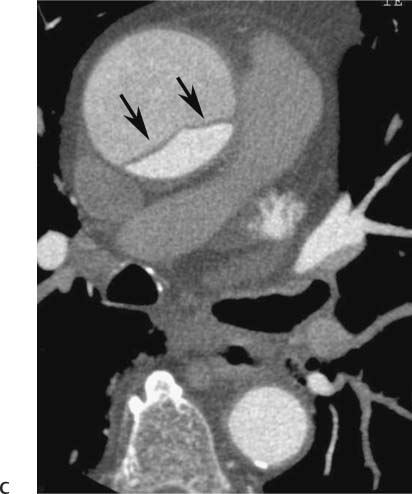
Follow-up
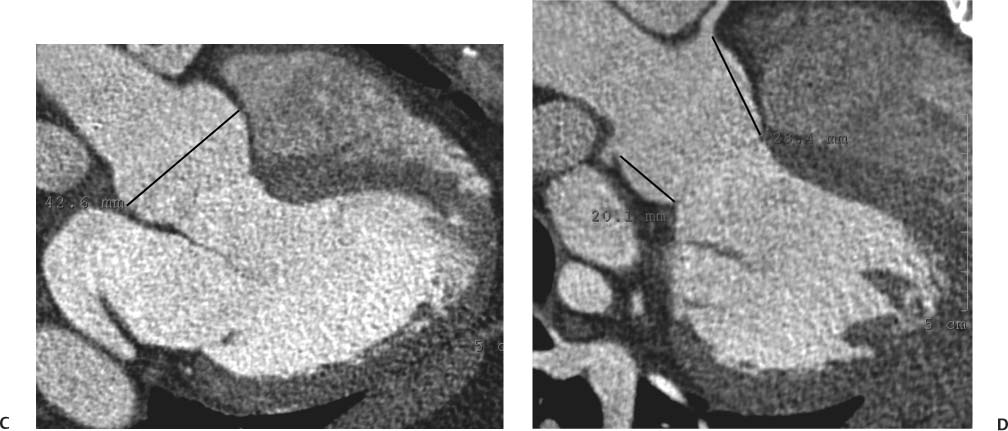
 Sinus of Valsalva Aneurysm
Sinus of Valsalva Aneurysm
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree