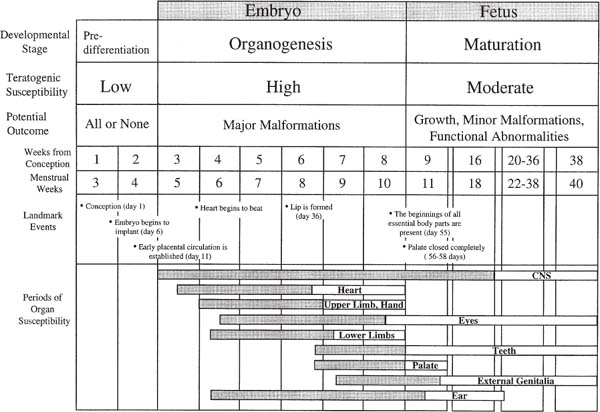
20 Teratogen Exposure The true risks of teratogen exposure are usually poorly understood by patients, and even by the physicians, nurses, and sonologists who attempt to provide counsel. Such misunderstandings can lead to both false assurances and false alarm, both unfounded dismissiveness and unwarranted anxiety. Teratogenesis results from genetic and environmental factors that singly or in concert alter the normal development of the embryo. Those environmental agents that can cause developmental abnormalities include drugs, chemicals, infection, procedures, radiation, and hyper-thermia. Genetic aberrations are determined before conception, or at least differentiation, and exert a preemptive governing effect on development. Considering that every neonate has at least a 5% risk of having a serious congenital abnormality, and that environmental teratogens account for ~10% of nongenetic human malformations, it is apparent that teratogenesis is a field of considerable importance for to those involved with prenatal diagnosis.1,2 There are four cardinal principles of teratogenicity.3,2 1. Teratogens exert their effects idiosyncratically across individuals of a population and between species. The expression of teratogenic effects is determined by a multitude of factors that interact in complex and unpredictable ways. The genetic constitutions of mother and fetus interact to create a unique background of resilience and vulnerability to a teratogen. Indeed, only a small minority of exposed fetuses will exhibit any adverse consequences, and the range of phenotypic expression is extremely broad.3 Such variable susceptibility is not well understood. It is nonetheless incontrovertible that only small proportions of fetuses exposed to hydantoins or even thalidomide develop malformations. Some mothers can consume alcohol throughout pregnancy without apparent ill effect to the fetus, whereas others imbibe sparingly and their offspring suffer grievously.3 Teratogenic effects can be further modified by the size, metabolism, parity, age, social class, and nutritional state of the mother, as well as the ethnicity, race, and gender of the baby.5 In addition, numerous environmental factors, such as season, temperature, and geography, have been shown to potentially influence the incidence of malformations.4 Interspecies differences are commonplace.6 Though animal studies are invaluable for the investigation of mechanism and pathogenesis, the results of these studies are not always directly transposable to the human case. This was most tragically demonstrated in the case of thalidomide, where tests on rodents indicated the benignity of the drug prior to its release, with devastating consequences for thousands of human babies. Unfortunately, this event has prejudiced some against all animal studies, a position that is excessive, unreasoned, and ultimately dangerous. 2. Susceptibility of a developing fetus to a teratogenic agent depends on the developmental stage of the fetus at the time of exposure. The developmental effects of a teratogen exposure can include death, malformation, growth disturbance, and functional disorder. Three important stages are usually distinguished: predifferentiation, embryonic, and fetal (Fig. 20–1). The first stage, encompassing the 2 weeks from fertilization to early implantation, is a period before cellular differentiation has occurred in the embryo and before the establishment of a placental connection to the blood supply of the mother. The embryo is relatively invulnerable to most teratogens during this period. Unless an agent can reach the embryo through the mucous blanket of the maternal genital tract or by other means independent of the maternal blood system, the embryo will not be affected. Those agents—such as radiation—that are not delivered by the maternal blood supply will result in embryonic death if significant cell loss or chromosomal defects are produced. Countervailing these effects is the plasticity of omnipotential embryonic cells, which can mount a potent reparative response. If affected at all, the embryo will tend to either recover or die. This binary response has been referred to as the “all or none” phenomenon. This is not to say that malformations cannot occur in this period, only that there is a propensity toward embryolethality rather than surviving malformed embryos. The second stage of development extends from roughly the third to the eighth week. This is the embryonic period when organogenesis occurs. During this stage, cells take on specific roles, grouping together to form organs at prescribed critical periods of formation.9 Each organ, then, has a window of particular vulnerability to teratogenic insult. Unfortunately, this period of greatest teratogenic susceptibility begins before most women know themselves to be pregnant, and sometimes even before the condition of pregnancy can be reliably ascertained. 20–1 Human embryonic development. Periods of greatest sensitivity (shaded) and lesser sensitivity to malformation are shown for individual organs. (This chart is modified, from The causes of human congenital malformations. In: Moore KL, ed. The Developing Human: Clinically Oriented Embryology. 4th ed. Philadelphia: 1988:131–158; and Risks. In: Kelly-Buchanan C, ed. Peace of Mind during Pregnancy. Philadelphia: Dell; 1989:7–26) The last stage of development, known as the fetal stage, begins after the ninth week of gestation. With the important exceptions of the genitourinary system, the palate, and the brain, most organs have formed and thereafter proceed to mature, function, and grow. Teratogenic insults during this period can lead to growth failure and eventual disturbances of behavior or fertility. Examples of behavioral teratogenicity include the hyperactivity, inattention, and tremulousness of children of narcotic-addicted mothers; the mental retardation of children of women exposed to anticonvulsants, alcohol, and lead; and the abnormal reflexes of children of mothers exposed to methyl mercury.10 3. Teratogenic induction is a threshold phenomenon. A dosage threshold must be exceeded for irreparable damage to be done. This can occur either as a single large dose, or as repeated chronic exposure, and the interaction of two or more drugs or chemicals may be synergistic. Equally important, especially for the parents, is the inverse formulation of this principle: There is a level below which no embryopathic effects can be measured.11 The concept of a placental barrier has been debunked as fallacious: Most drugs and chemicals readily reach the fetus in significant concentration soon after administration.12 4. Typically, teratogens cause characteristic patterns of malformations rather than single defects. The final pattern of malformation represents not only the consequences of initial injury mediated through cell death and disruption of cell growth and metabolism, but also that of secondary reparative and regenerative processes. For example, the intraabdominal and intracranial calcifications characteristic of some viral infections represent a reparative response to initial injury. Establishing the safety or risk of drugs is a daunting task fraught with many methodological problems. Even known teratogens do not affect the great majority of exposed fetuses because teratogenic effects are idiosyncratic and mitigated by a wide range of genetic, environmental, developmental, and physiological factors.13,2 Indeed, only a few of the most potent teratogens increase the background malformation rate by a factor of two or more.13 To illustrate, if the background rate is 3%, then at least 220 exposed fetuses (and a similar number of nonexposed fetuses) would be needed to establish the risk of an agent that increases the malformation rate by a factor of 2.5, with a statistical power of 80%.13 This is the level of teratogenicity seen only with the most severe and virulent agents, such as thalidomide and isotretinoin. Teratogens are identified and characterized on the basis of case reports of individuals, epidemiological studies of populations, and controlled laboratory studies on animals. Clinical case reports serve to provide working hypotheses for the teratogenicity of an agent but cannot establish a quantitative index of risk. Case reports are rarely persuasive in themselves, except perhaps if the agent is a usually potent teratogen, few women have been exposed, and the consequent malformation is rare.13 Such was the case with warfarin, diethylstilbestrol, and isotretinoin.13 In most cases, however, the association of a congenital malformation and an environmental exposure is purely coincidental, especially if the exposure or the malformation is common. Such was the case with Bendectin and other drugs (Table 20–1, Exonerated drugs). An apparent association may result from the convergence of the baseline rate of malformations in the general population (estimated between 1 and 5%) and the widespread use of the agent. Epidemiological studies are necessary to establish risk when the agent is widely used, the malformation is common, and the teratogenic potency of the drug is relatively weak. There are two types of epidemiological studies: case control and cohort. Case-control studies are retrospective: Investigators look backward into the histories of children with and without the malformation to determine if children with the malformation were more likely to have had an exposure. Cohort studies are prospective: Investigators look forward from records of exposure to rates of malformation. Retrospective studies can be skewed by recall or ascertainment biases. Prospective studies can be confounded by any number of incidental factors (such as the maternal illness that occasioned the exposure) if these factors are not distributed randomly between exposed and nonexposed groups.15 Though it is true that the results of animal studies cannot be extrapolated with certainty to humans, animal studies have proven invaluable in assessing developmental risks and preventing the catastrophic exposures in the human population.13 Every true human teratogen has had parallel effects in animals, with two exceptions—thalidomide and misoprostol. In the case of isotretinoin, animal studies prevented a human disaster similar to that of thalidomide.13 Indeed, controlled experimentation across a range of doses during the period of organogenesis can only be performed in animal models. Epidemiological studies alone are only conclusive once the agent has damaged many children. Still, caution is required in the interpretation of animal studies: dosages may be many times greater than those likely to occur in humans, and maternal toxicity may contribute to the observed effect. Further, teratogenic effects seen at high doses in animals may not be present at clinical dosage levels in humans (as is the case with benzodiazepines, salicylates, and glucocorticoids).
Clinical Principles of Teratogenicity
Identifying Teratogens
Date Discovered | Drug | Approximate Number Cases Malformed |
1903 | Antithyroid compounds | 140 |
1950 | Aminoglycoside antibiotics | 60 |
1952 | Anticancer agents | 50 |
1953 | Androgenic hormones | 250 |
1956 | Tetracyclines | Thousands |
1961 | Thalidomide | 7700 |
1963 | Phenytoin | Hundreds |
1965 | Hypervitaminosis A | 20 |
1966 | Coumarin anticoagulants | 55 |
1967 | Alcohol | Thousands |
1970 | Methadione anticonvulsants | 40 |
1970 | Lithium | 25 |
1970 | Diethylstilbestrol | Hundreds |
1971 | Penicillamine | 5 |
1976 | Primidone | 25 |
1982 | Valproic acid | 100 |
1983 | Vitamin A analogues | 115 |
1987 | Cocaine | Hundreds |
1988 | Carbamazepine | 70 |
Adapted from Turner GM, Twining P. The facial profile in the diagnosis of fetal abnormalities. Clin Radiol 1993;47:389–395 and from Schardein JL. Chemically Induced Birth Defects. 2nd ed. New York: Marcel Dekker; 1993:398641.
Meta-analyses combine similar studies to increase sample sizes. Such analyses, though, require that the combined studies be comparable in quality and methods, which may not always be the case. Further, there tend to be fewer studies in the literature that do not find an association because publication bias against negative studies leads to unbalanced reporting.
In the end, several conditions must be satisfied to establish the teratogenicity of an agent from available evidence. The evidence must be reproducible, consistent, and biologically plausible.16 Epidemiological studies should be independent and demonstrate similar effects. Animal studies have greater credence if the exposure is comparable in dose and route of exposure as in humans, and if the species is phylogenetically close to humans. And finally, the teratogenic effects must be biologically plausible: effects should be related to dose, timing during development, and presence at susceptible sites within the fetus.
Ultrasound Evaluation
The sonologist evaluating a patient with teratogen exposure will need to first assess the developmental risks of the exposure. This requires interviewing the patient to determine the agent, the dose, and the timing of the exposure. Specific agents tend to produce specific patterns of abnormality. Both the incidence and the severity of malformations tend to increase with the dose (Table 20–1). As stated earlier, timing is crucial. Teratogenic exposures in the first 2 weeks following conception are less likely to result in malformation in embryos that survive. Exposures occurring during the embryonic stage should be placed as precisely as possible in those critical 6 weeks so that the examination can be concentrated on those organs most susceptible to injury (Fig. 20–1).
Once risk is assessed, the sonologist will likely benefit from any of several information or consultative resources. Online databases provide perhaps the most current and readily accessible summaries of the medical literature and estimates of risk. Particularly valuable services are provided by the ReproTox system,17 the Motherisk program (416–813–6780, www.motherisk,org), and the Organization of Teratogen Information Services (OTIS, 801–328–2229, http://orpheus.ucsd.edu/ctis). The OTIS Web site has very good fact sheets for many drugs that can be downloaded and printed in English, Spanish, and French, and which are written in accessible lay language.
Other useful electronic resources that can be accessed online are the Teratogen Information System (TERIS),18 ReproRisk, and Shepards Catalog of Teratogenic Agents.19 If an unusual abnormality or pattern of abnormalities is found in a fetus, possible etiologies can be found in such databases as POSSUM (Pictures of Standard Syndromes and Undiagnosed Malformations), Platypus, and the London Dysmorphology Database. Finally, there are several excellent books and review articles that list teratogens and their described effects, though these will be variably current.20–25 Though dated, the book Peace of Mind during Pregnancy by Christine Kelley-Buchanan is particularly useful for counseling patients because it is written in direct and clear language that will be accessible to most patients.26
Ultimately, the sonographic study of a pregnancy with a known teratogenic exposure will need to be more comprehensive and meticulous than routine studies. In addition to the routine fetal survey, additional scrutiny will need to be directed to the face, calvarium, spine, heart, limbs, hands and feet, and genitalia, as well as to the measures of fetal growth, amniotic fluid volume, and placental function. Knowing the probable effects of a teratogen will help focus this rather formidable and extensive survey.
This said, one small study of 126 pregnancies studied by ultrasound for teratogen exposure found only one structural abnormality.27 This study, however, made no accounting of the magnitude or the timing of the exposures and included a broad miscellany of agents, most of which contribute five or less episodes of exposure each. As discussed earlier, the likelihood that such a study would be able to identify the effects of even the most potent teratogen is extremely small. The value of this study is highly questionable, and its conclusions could be misleading.
Observed effects can be organized into four basic categories: malformation, growth retardation, death, and functional impairment.4 Of these, growth disturbances are the most common and sensitive signs of exposure.8
Craniofacial anomalies can generally be depicted with routine imaging, though more subtle abnormalities may require morphometric analysis.28 Standardized linear measurements have been proposed to describe the growth of normal craniofacial structures, including the mandible.29–31 Even so, the morphometric approach has not been widely adopted in prenatal laboratories, partly due to the problem of obtaining reproducible planes of imaging and partly due to the inherent imprecision of the relatively small measurements. Nonetheless, these techniques have been well established in infants and children32 and may yet prove to be transposable to the fetus.
Functional and neurobehavioral effects may be revealed in observations of fetal breathing, movement, behavior, and adaptability to stimulus. The agitated behavioral state of a cocaine-exposed fetus may be the only detectable abnormality. Choanal atresia in a coumarin-exposed fetus could theoretically be detected by an abnormal pattern of fetal breathing visible with color Doppler techniques. In cases of misoprostol exposure, Doppler techniques can demonstrate increased resistive indices in the uterine artery, an important indicator of compromise of the uteroplacental perfusion that is the presumed mechanism of fetopathy. Such observations test the limits of the capabilities, skills, and patience of the examiner. The success of the enterprise, however, will finally depend on the ability of the sonologist to make subtle and careful observations.
Classes of Teratogens
Pharmaceuticals
Despite the widespread recognition that drugs taken during pregnancy could affect the fetus, recent studies have shown that drug use during pregnancy is, in fact, increasing.33 An average of 81% of pregnant women are exposed to drugs sometime during their gestation, and the average number of drugs taken is approximately six.34 Nonetheless, apart from exposures to established teratogens, the risk of a major fetal malformation following general maternal drug use is low: Case-control studies from large birth registries suggest an odds ratio of 1.2.35
The myth that the uterus is a privileged sanctuary for the fetus, largely impervious to the noxious agents of the maternal world, was shattered by the thalidomide catastrophe. To be sure, an association had already been reported between maternal rubella infection and severe fetal malformations as early as 1941, but the extreme vulnerability of the fetus to drugs and chemicals had not been widely suspected.36–38 Indeed, essentially all drugs are transferred across the placenta to the fetus, with rare exception.33 In subsequent years a broad spectrum of drugs have come under closer scrutiny. Even so, fewer than 30 drugs have been identified as human teratogens and many of these are no longer in clinical use (Table 20–1).13 More recent entries to Table 20–1 would include angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor antagonists, fluconazole, systemic corticosteroids, misoprostol, and methimazole.39
It is equally important to recognize that several drugs had been initially identified as teratogens, but were subsequently shown not to be in larger and better-controlled studies.13 These include diazepam (Valium) (reported to cause oral clefts); oral contraceptives (pseudohermaphroditism, various malformations in the VACTERL spectrum); spermicides (limb defects, tumors, hypospadius); salicylates (cleft palate, congenital heart disease); and Bendectin (cardiac and limb defects).13
Thalidomide
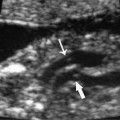
Once a popular tranquilizer outside the United States, thalidomide damaged an estimated 7700 children before its teratogenic effects were discovered.40,2 Malformations occur in roughly 20% of exposed fetuses and include limb reduction defects, esophageal and duodenal atresia, tetralogy of Fallot, renal agenesis, facial hemangiomata, and anomalies of the external ear (Fig. 20–2). Following the catastrophe, the use of thalidomide as a sedative ended in the 1960s, but has been recently reinstituted in clinical practice for the treatment for leprosy and multiple myeloma. The drug is also being studied for use with several malignant diseases, such as myelofibrosis, renal cell cancer, prostate cancer, and Kaposi sarcoma.42
Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Antagonists
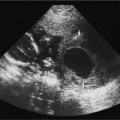
Used for the treatment of hypertension, these drugs act on the renin-angiotensin system by different mechanisms to reduce angiotensin II production. Angiotensin II, though, is also a growth factor for the fetal kidney and is important for nephrogenesis.15 These drugs do not appear to have teratogenic effects in the first trimester of pregnancy, but have been associated with renal dysgenesis, fetal oliguria (and oligohydramnios), skull defects, fetal growth restriction, and death when the fetus is exposed in the second and third trimesters43,2 (Fig. 20–3). It has been suggested that hypotension is an important mechanism for the malformations.45 Serial fetal sonograms for amniotic fluid volume and fetal growth are indicated for exposures later in pregnancy.
Figure 20–2 Phocomelia and micromelia. The upper limb of this fetus (arrows) is markedly short with complete or partial absence of individual bones. Such an abnormality was found in babies of mothers who had taken the sedative thalidomide. Though no longer used as a sedative, this drug is being reintroduced as a treatment for skin disease.
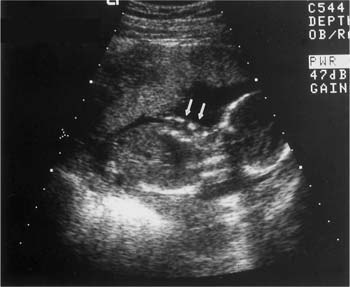
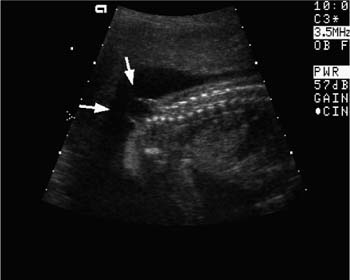
Figure 20–3 Renal dysgenesis. Echogenic and dysmorphic kidneys (arrows) are a feature of angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists, when exposure occurs in the second or third trimester.
Figure 20–4 (A,B) Terminal transverse defect in the upper limb (arrow). Limb defects can be the result of dysmorphogenesis (thalidomide, warfarin, phenytoin), or vascular disruption of the limb that had formed normally (misoprostol, chorionic villus sampling). (Courtesy of Carol B. Benson, M.D., Boston, MA)
Misoprostol
When used in the treatment of peptic ulcer disease, misoprostol (a prostaglandin E1 analog) was found to also cause endometrial bleeding.46 This led eventually to its use as an abortifacient in Brazil, where abortion is illegal and prescription drugs can be purchased over the counter. This confluence of factors led to the widespread popularity of the drug: fully 11% of women delivering in Rio de Janeiro in 1993 had used misoprostol in attempts to end their pregnancies.46 Unfortunately, misoprostol alone will induce abortion in only 11 to 20% of cases, which resulted in a large number of exposed fetuses.47 Though the frequency of malformation has not been established prospectively, one retrospective case-control study found an odds ratio of 29.7 (95% CI 11.6 to 76.0) comparing mothers who had used misoprostol in the first trimester to mothers who had given birth to infants with spina bifida.48
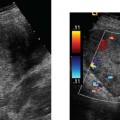
Misoprostol causes intense uterine contractions followed by bleeding.49 This is postulated to lead to hypoper-fusion of the fetus, and ischemia and infarction in tenuously perfused areas served by end arteries (limbs, brain stem, spinal cord, intestine, tongue). The resulting vascular disruption defects include cranial nerve hypoplasia and terminal transverse defects in the arms and legs (and other limb abnormalities) (Fig. 20–4A,B). This combination of abnormalities has been referred to as the Mobius sequence.46,2
Teratogen-induced limb defects, therefore, can be either the result of limb dysmorphogenesis (thalidomide, warfarin, phenytoin), or vascular disruption of a limb that had formed normally [misoprostol, chorionic villus sampling (CVS)].46 The pathogenic hypothesis for misoprostol is supported by Doppler ultrasound studies showing an increase in the resistive indices of the uterine arteries in women taking misoprostol.50
Thyroid Agents
Antithyroid agents such as iodine-131 readily cross the placental membrane and are taken up by the fetal thyroid with an avidity that exceeds even that of the mother.51 Methimazole, carbimazole, and propythiouracil (PTU) can cause fetal goiter and hypothyroidism if used after 10 weeks gestation when the fetal thyroid begins to concentrate iodide, though there is usually a return to the euthyroid state within days or weeks after birth.52 The risk is probably minimal with methimazole and carbimazole and small with PTU. Prenatal ultrasound evaluation for fetal thyroid enlargement is indicated. Both methimazole and carcimazole have been associated with scalp defects (aplasia cutis), and therefore PTU is currently the drug of choice during pregnancy.52
Thyroid replacement agents, by distinction, are predominantly protein bound and, therefore, do not readily cross the placenta.53 These agents are considered to have a low teratogenic potential.
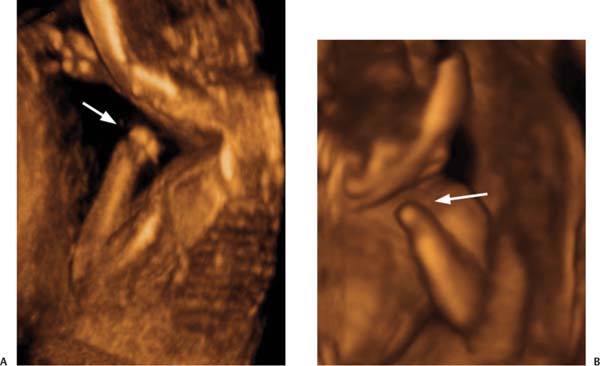
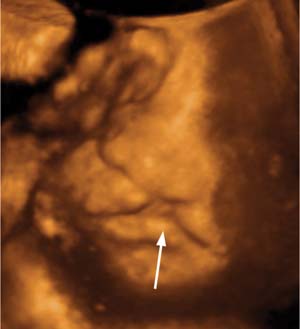
Figure 20–5 Cleft lip and palate. A complete unilateral defect (arrow) is seen in this modified coronal view. This finding can be found following exposure to hydantoins, valproic acid, trimethadione, retinoids, cyclophosphamide, ethanol, cocaine, glucocorticoids, and hyperthermia. (Courtesy of Carol B. Benson, M.D., Boston, MA)
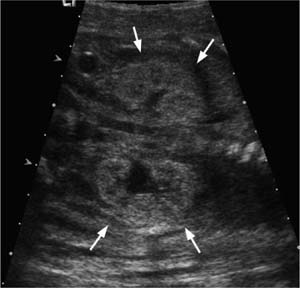
Figure 20–6 Neural tube defect. Spinal dysraphism and a meningomyelocele (arrows) is demonstrated in this fetus. This abnormality is a prominent feature of embryopathy resulting from valproic acid, carbamazepine, methotrexate, lithium, ethanol, and hyperthermia.
Anticonvulsants
The rate of congenital malformation is increased 2 to 3 times for epileptics receiving anticonvulsant therapy over nonepileptics or epileptics who were not medicated.54,2 Anticonvulsant therapy has been particularly implicated in the induction of cleft lip–cleft palate, and congenital heart disease (Fig. 20–5
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree