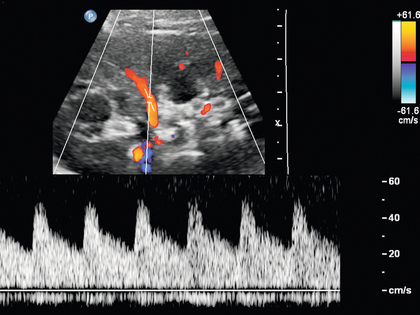
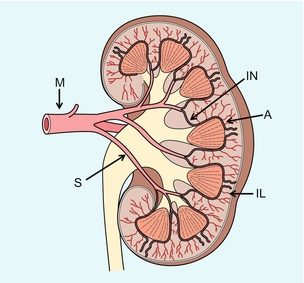
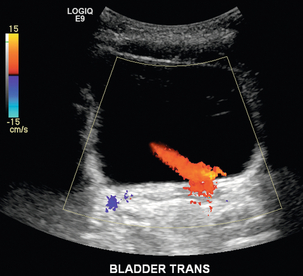
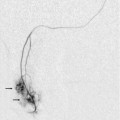
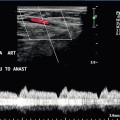
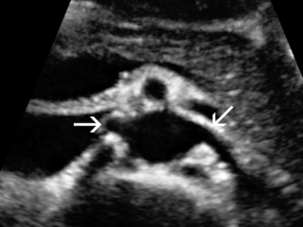
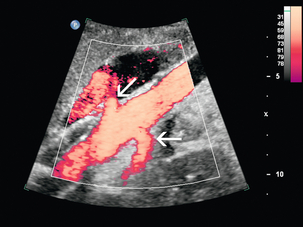
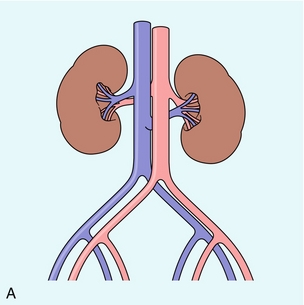
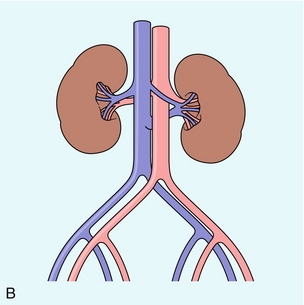
Chapter 9 Common clinical indications for renal ultrasound include renal insufficiency and renal failure. Specifically, the request to exclude renal obstruction as the aetiology of acute renal failure leads the list. Doppler ultrasound is not routinely performed to evaluate acute renal failure but may be prompted by certain clinical indicators (Box 9-1) or greyscale findings. Colour and spectral Doppler is more commonly used in the native kidneys for evaluation of unexplained or uncontrolled hypertension caused by renal artery stenosis (RAS) or for determination of vessel patency. In hypertensive patients, some authors suggest Doppler should be reserved for those patients with a strong clinical suspicion for RAS who are likely to benefit from intervention.1 As will be shown, there are many other vascular abnormalities than can be demonstrated by Doppler, and these can present with a wide variety of symptoms or signs. Doppler evaluation of the renal arteries should not occur without a thorough greyscale examination of the kidneys. Greyscale imaging can provide useful information about renal size and cortical thickness and should be part of the initial series of images. For Doppler image acquisition, a preliminary scan of the abdominal aorta is performed with colour Doppler in the transverse plane beginning at the level of the superior mesenteric artery (SMA) to locate the main renal arteries, which typically originate within 2 cm of the SMA (Fig. 9-1). Transverse images may be obtained from a midline approach with the patient supine or rolled into the left lateral decubitus position. The imager can localise the right renal artery passing posterior to the IVC then rotate the transducer while maintaining the artery in view. Also, the transducer can be placed longitudinally lateral to the rectus muscle resulting in a ‘banana peel’ image (Fig. 9-2), in which the aorta is the banana and the renal artery is the banana skin on each side, being peeled off the banana. If the main renal arteries cannot be demonstrated from the midline approach, a right or left lateral approach is used to follow each artery centrally from the renal hilum. Regardless of the technique used to identify the renal artery origin, the entire main renal artery should be visualised sonographically. Lack of visibility of even a 10 mm segment of main renal artery will limit the sensitivity of the direct method for RAS. This is especially relevant in younger patients in whom fibromuscular dysplasia is a concern; stenosis in these patients may not be near the renal artery origins (described later). As part of the direct Doppler evaluation, the peak systolic velocity with angle correction should be measured at the renal artery origin, mid and distal artery, and at any region of turbulent disorganised flow with aliasing on colour Doppler. FIGURE 9-1 Normal renal arteries. Greyscale transverse image of the aorta demonstrates normal origins of the renal arteries (arrows). FIGURE 9-2 Normal renal arteries. Longitudinal power Doppler shows the aorta and both renal artery origins in normal location (arrows) with appearance termed as a ‘banana peel’. Accessory renal arteries occur commonly (approximately 30% of kidneys), but are not always demonstrated sonographically. In fact, studies suggest that only 21–41% of accessory renal arteries are visualised by Doppler evaluation.2,3 This low success rate has prompted some individuals to argue that sonographic evaluation for RAS is not sensitive enough as a screening study. However, Bude et al. found that less than 1% of accessory renal arteries were the only stenotic artery,4 which essentially negates the significance of not visualising an accessory renal artery. When the main renal artery is not well seen in its entirety, evaluation of the segmental intrarenal arteries may allow a non-diagnostic direct examination to become diagnostic for RAS.5,6 We always examine the segmental arteries even when the main renal arteries are well seen, because the segmental artery waveform morphology may be useful in detecting concomitant renal parenchymal disease. Characteristics of the spectral Doppler tracing in normal segmental intrarenal arteries should include rapid upstroke to an early systolic peak with gentle decrease in flow velocity during late systole and diastole (Fig. 9-3). Persistent antegrade flow throughout the cardiac cycle should be present without return to baseline. The resistive index (RI), calculated as: is a common parameter for characterisation of arterial flow. The RI is inversely proportionate to the relative amount of diastolic flow. For instance, an end diastolic velocity that is 20% of the peak flow will result in RI of 0.80. The upper limit of RI in normal adults has been reported as less than 0.70,7 but concern for pathology is not often raised until the RI is 0.75–0.80, or higher. Furthermore, the RI may be affected by other factors such as heart rate, Valsalva, and arterial compliance. In fact, RIs greater than 0.70 are common in elderly patients.8 The impact of systemic vascular disease in chronic renal dysfunction is significant. It has been recently suggested that the renal RI measurement does not distinguish local from systemic vascular damage. A new potential ultrasound measurement, the difference of RIs between the spleen and kidney, may allow more specific evaluation of renal parenchymal damage.9 However, this study has not yet been further validated or widely applied in practice. The renal arteries typically arise from the abdominal aorta caudal to the level of the SMA. The right renal artery usually originates from the anterolateral aspect of the aorta, while the left renal artery usually originates from the posterolateral aspect. As noted earlier, approximately 30% of patients will have more than one renal artery.10 Accessory renal arteries usually arise from the aorta caudal to the main renal artery to supply the renal lower pole, but occasionally will course cranially to supply the upper pole. Rarely, accessory arteries may arise from an iliac artery or even the SMA. Renal anomalies such as horseshoe or pelvic kidney almost always have multiple renal arteries, which may arise from the aorta or iliac arteries. The branching pattern of the renal arteries progresses symmetrically to the renal cortex (Fig. 9-4). Segmental branches arise from the dorsal and ventral rami and run along the infundibulae before dividing into interlobar arteries. These interlobar arteries course between the pyramids, and then branch into arcuate arteries, which run along the bases of medullary pyramids. Within the cortex, small interlobular arteries course outward toward the surface of the kidney. The renal venous anatomy parallels the arterial anatomy. Normal venous flow on spectral Doppler has a relatively low velocity. Its waveform is driven by right atrial activity. Accessory left renal veins are less frequent than accessory renal arteries; however, accessory right renal veins are quite common. Left venous anomalies may be seen in approximately 11% of patients.11 Variants most commonly include the retroaortic and circumaortic renal veins (Fig. 9-5), and these may be clinically relevant even beyond filter placement. In a recent study by Karazincir et al., the incidence of retroaortic left renal vein was found to be significantly higher in patients with varicocele, compared with controls12 (see Fig. 9-24 in the varicocele section). FIGURE 9-5 Renal vein variants. Line drawings demonstrate the anatomic appearance of a retroaortic (A), and circumaortic (B) left renal vein. Doppler can play a supportive role in the diagnosis or exclusion of renal obstruction in patients with acute renal failure. Identification of a dilated renal collecting system is fairly easy with ultrasound. The difficulty, however (in the absence of prior examinations) is the differentiation of an acutely obstructed high-pressure system versus that of a low-pressure, chronically dilated system. It has been suggested that elevated resistive index may help differentiate between severe acute urinary obstruction and chronic dilatation.13–15 The RI of the obstructed kidney may be elevated relative to the normal contralateral kidney. An RI difference of greater than 0.10 between the non-obstructed and obstructed kidney is the suggested threshold for diagnosis of acute obstructed uropathy. However, intra-renal autoregulatory hormonal systems counteract the mechanical effect of the high-pressure collecting system pressing upon the parenchyma. This rapidly modifies the resistance to flow, reducing sensitivity of the test. In the setting of partial obstruction or less severe obstruction, this finding also lacks sensitivity.16,17 As an aside, in cases of chronic renal disease without obstruction, elevated RI > 0.80 has been shown to be associated with worsening renal function and mortality.18 Another Doppler tool to assist in the evaluation of urinary obstruction can be performed within the bladder. In cases with suspected renal obstruction, sonographic evaluation for a ureteral jet should be a component of the renal ultrasound examination (Fig. 9-6). Although entry of urine into the urinary bladder is not synchronous, demonstration of three or more ureteral jets by Doppler on one side without a single pulse of flow from the contralateral side implies obstruction of the non-pulsing ureter.
![]() The Kidneys
The Kidneys
Renal Vascular Doppler Ultrasound
CLINICAL CONSIDERATIONS
TECHNICAL CONSIDERATIONS
Main Renal Artery Evaluation
Segmental Intrarenal Artery Evaluation
Anatomy of the Native Kidneys
ARTERIAL ANATOMY
VENOUS ANATOMY
Renal Failure and Obstruction
Radiology Key
Fastest Radiology Insight Engine