The Mediastinum
Srinivas Tummala
Janet E. Kuhlman
S. Tummala: Department of Radiology, University of Wisconsin Hospital and Clinics, Madison, Wisconsin 53792-3252. J. E. Kuhlman: Department of Radiology, University of Wisconsin Medical School, Madison, Wisconsin 53792-3252.
INTRODUCTION
Primary mediastinal tumors affect patients of all ages. They encompass a long list of histologically diverse lesions that can arise from a wide variety of mediastinal structures.10 Over the past 10 years, advances in diagnostic imaging, biopsy techniques, and cytologic techniques have enhanced the physician’s ability to localize, identify, and determine the extent of involvement of mediastinal masses.
According to Hoffman et al,10 approximately 50% of mediastinal tumors are discovered incidentally on chest roentgenograms. Such studies provide limited information regarding the morphology and extent of the lesion but are important in localizing the mass to one of the mediastinal compartments,12 which helps to narrow the diagnostic possibilities.
Computed tomography (CT) has proved to be the most efficacious study to evaluate a mediastinal abnormality observed on plain films. It provides excellent contrast discrimination between fat, air, calcification, and soft-tissue structures and eliminates the problem of superimposition of structures seen with the chest radiograph. Furthermore, with the use of intravenous contrast agents, CT can further define vascular structures and better determine the relative vascularity of structures other than vessels.8,10
Magnetic resonance imaging (MRI), on the other hand, has a limited role compared with CT. In evaluating mediastinal tumors its limitations include decreased spatial resolution, prolonged scanning times, and relatively highcost.10,12 However, exceptions do exist. MRI allows improved imaging in nonaxial planes, identification of vascular lesions without the use of intravenous contrast material, and somewhat improved soft-tissue differentiation compared with CT.12,13 In addition, it allows better evaluation of the hila and lung apices without contrast and is clearly superior to CT for evaluation of cardiac and paracardiac masses.8,10
Anatomy
The mediastinum is defined as the space within the thoracic cavity that is bounded anteriorly by the sternum, posteriorly by the vertebral bodies, superiorly by the thoracic inlet, inferiorly by the diaphragm, and laterally by the parietal pleura.10,21 Anatomically, the mediastinum is divided into compartments by drawing a line from the sternal angle to the fourth thoracic intervertebral space. The area above this line is called the superior compartment, and the area below is called the inferior compartment. The inferior compartment is then further subdivided into anterior, middle, and posterior compartments. From a practical standpoint, however, most clinicians divide the mediastinum into three compartments as viewed on a lateral chest radiograph. The superior compartment is omitted and the anterior, middle, and posterior compartments are defined as extending vertically from the thoracic inlet down to the diaphragm.3,7,10 Some of the diseases and tumors that can affect these compartments are reviewed here.
Inflammatory Diseases
Acute Mediastinitis
Acute mediastinitis is a rare but life-threatening condition. Survival requires prompt diagnosis and treatment, because mortality can range from 50% to 75% when surgery is delayed. If therapy is instituted within 24 hours of the precipitating event, mortality can be reduced to 25% or less.3,20 The most common causes are esophageal rupture and postoperative infection related to median sternotomy.1,8 Esophageal perforation may occur secondary to penetrating chest trauma, esophageal instrumentation (endoscopy, biopsy, dilatation, or stent placement), or ingestion of a foreign body or corrosive substance. In addition, malignancy and Boerhaave’s syndrome are additional possibilities. Boerhaave’s syndrome is often caused by prolonged vomiting that results in a tear involving the left posterolateral aspect of the esophagus
just above the gastroesophageal junction. A less common but still important cause of acute mediastinitis is the spread of infection from adjacent structures, such as the head and neck, lungs, pericardium, and spine.
just above the gastroesophageal junction. A less common but still important cause of acute mediastinitis is the spread of infection from adjacent structures, such as the head and neck, lungs, pericardium, and spine.
Clinically, the presentation can be dramatic and is characterized by fever, chills, dysphagia, chest pain, and often evidence of septic shock. On chest auscultation, an apical systolic crunching sound, known as Hamman’s sign, may be present.3
Often, the radiographic diagnosis is straightforward. Chest radiography may demonstrate pneumomediastinum, a widened superior mediastinum, or pleural effusions, with the latter two findings present in 66% and 50% of patients, respectively.3 In Boerhaave’s syndrome the pleural effusion commonly occurs on the left, often with left-lower-lobe consolidation. Other features better delineated by CT include obliteration of normal mediastinal fat planes, localized mediastinal fluid collections, and abscess formation.
If esophageal perforation is suspected, an esophagram with Gastrografin or a nonionic contrast agent should be performed as soon as possible to detect extravasation of contrast material into the mediastinum and possibly to pinpoint the site of perforation. Brant and Helms3 stated that the sensitivity of an esophagram is highest if it is obtained within 24 hours after the perforation occurs.
A separate but related topic concerns the complications of median sternotomy. Often, benign postoperative changes are difficult to distinguish from changes caused by acute mediastinitis. For example, on CT, postoperative granulation tissue can appear similar to a retrosternal hematoma or abscess. In addition, the direct effects of a median sternotomy may be impossible to separate from sternal osteomyelitis.1 Clearly, the history and clinical findings are most helpful in differentiating these conditions.
Chronic (Fibrosing) Mediastinitis
Chronic fibrosing mediastinitis is an extensive fibrotic reaction involving the mediastinum. It most commonly occurs secondary to histoplasmosis, although other causes include tuberculosis, syphilis, drug (methysergide) therapy, and radiation therapy.17 Rarely, lymphatic obstruction, nocardia, or sarcoidosis may be responsible. An idiopathic type, potentially autoimmune in nature and sometimes combined with retroperitoneal fibrosis, has been described.1
The typical patient tends to be between 20 and 40 years of age. The patient may be asymptomatic or present with symptoms related to compression or occlusion of mediastinal structures. These symptoms most commonly include cough (45%), dyspnea or shortness of breath with exertion (42%), and superior vena cava syndrome (39%).17 Other less common findings may include chest pain, recurrent pulmonary infections, hemoptysis, dysphagia, or hoarseness.
Radiographically, plain films may be normal or display slight mediastinal widening with preference for the right paratracheal area. If a pulmonary artery is severely compromised, unilateral pulmonary oligemia may be identified. In addition, bronchial, venous, or lymphatic obstruction may lead to atelectasis or pulmonary consolidation.1 If the cause is histoplasmosis, then mediastinal or hilar lymphadenopathy with “popcorn” calcifications may be present.
At CT, a localized mediastinal soft-tissue mass with calcification or more diffuse soft-tissue thickening throughout the mediastinum may be seen. In a patient with the appropriate clinical history, the diagnosis of fibrosing mediastinitis can be strongly suggested. Often in these instances, tissue sampling may not be required.1,8,17
A clear advantage of MRI over CT in this disorder is that it can often differentiate adenopathy caused by fibrosing mediastinitis from that caused by malignancy.1,8 For example, MRI usually demonstrates a signal intensity of the fibrotic tissue on T2-weighted images that is less than that of muscle. In contrast, lymphomas and carcinomas have higher signal intensity than muscle on T2-weighted images.1,8,9,17 A disadvantage of MRI, however, is that it does not reliably demonstrate calcifications, which, when present, greatly aid in making the diagnosis.
Miscellaneous Conditions
Mediastinal Lipomatosis
Mediastinal lipomatosis is a benign asymptomatic condition that is characterized by excessive accumulation of fat in the mediastinum. It is commonly caused by obesity, Cushing’s disease, or exogenous corticosteroids, but in approxi mately 50% of cases there is no predisposing factor.1,3,6,8 Plain radiographs typically demonstrate a symmetrically widened superior mediastinum with a lobulated border. Unlike tumor or hemorrhage in the mediastinum, there is no tracheal narrowing or deviation. Additional evidence of fat deposition includes enlargement of the epicardial fat pads, thickening of fat along the lateral chest walls, or accumulation in the paraspinal regions.1,3 CT usually provides the definitive diagnosis, although it should rarely be needed. It demonstrates uniform fat density in the range of −100 Hounsfield units (HU) in the upper mediastinum without evidence of tracheal displacement or compression.8 Furthermore, if the fat demonstrates heterogeneity (e.g., strands of soft-tissue density within it) or has a higher density than expected, other conditions such as malignancy, hemorrhage, fibrosis, or infection should be considered.
Mediastinal Hemorrhage
Mediastinal hemorrhage is a condition that usually requires immediate attention. Injury to the mediastinal vessels from blunt or penetrating trauma is the most common cause of this condition. Blunt trauma from a motor vehicle accident usually results in shearing effects at the aortic isthmus. Penetrating trauma, which may be iatrogenic in nature, commonly occurs during attempts at central line placement. The usual nontraumatic
causes include bleeding from a coagulopathy or anticoagulant therapy and aortic rupture of a dissection or an aneurysm. Rarely, chronic hemodialysis, radiation vasculitis, or bleeding into a pre-existing mediastinal mass can cause mediastinal hemorrhage.1
causes include bleeding from a coagulopathy or anticoagulant therapy and aortic rupture of a dissection or an aneurysm. Rarely, chronic hemodialysis, radiation vasculitis, or bleeding into a pre-existing mediastinal mass can cause mediastinal hemorrhage.1
Patients may be asymptomatic or present with retrosternal chest pain which may radiate to the back. Often further workup leads to aortography, but CT and MRI also play important diagnostic roles, with the most appropriate study being determined by the clinical situation.
Plain films most commonly demonstrate widening of the mediastinum. If the blood is free flowing, it can track along the extrapleural space, giving rise to the so-called apical cap.1 In addition, the hemorrhage can track along the bronchovascular bundles and give rise to an appearance similar to pulmonary edema. This finding, however, is quite rare.
CT usually demonstrates fluid in the mediastinum which may be a localized collection or more diffuse tracking around the aorta and mediastinal vessels. With precontrast scans, high-density areas associated with fresh thrombus can be identified. Occasionally, a hematoma in the mediastinum can be difficult to differentiate from a mediastinal mass on CT. The proper clinical history may clarify this situation.1
ANTERIOR MEDIASTINUM
Lymphoma
The lymphomas are malignant neoplasms of lymphocytes and histiocytes mixed with non-neoplastic inflammatory cells.1 They are commonly classified into two types: Hodgkin’s (HL) and non-Hodgkin’s (NHL).
HL comprises approximately 20% to 40% of all lymphomas.3,4 It is differentiated histologically from NHL by the Reed-Sternberg cell. The Rye modification of the Lukes-Butler system divides HL into four subtypes (Table 32-1).1 According to this system, the nodular sclerosing subtype comprises 40% to 75% of cases and most commonly affects women.1
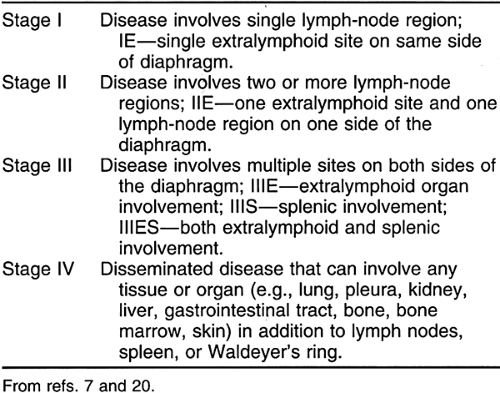
Traditionally, patients are staged by the Ann Arbor staging system (Table 32-2). Under this system patients are further subclassified as A for absence of, or B for presence of, fever, night sweats, weight loss, or pruritus that is not explained by other disease.
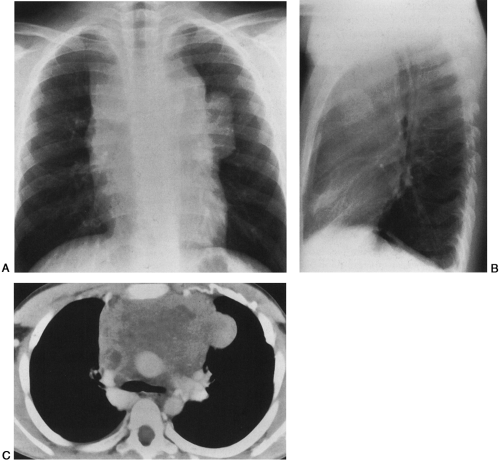
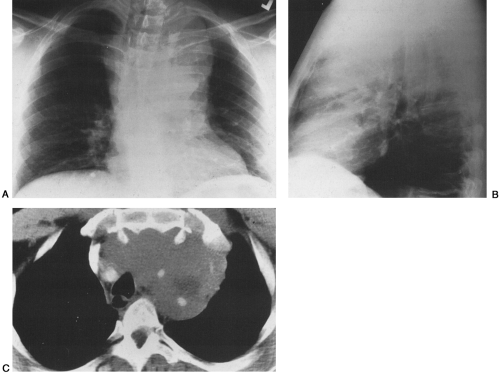
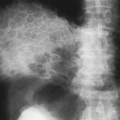
HL has a bimodal age distribution, affecting patients between 25 and 30 years of age and those older than 70 years of age. At presentation, 67% of patients have intrathoracic involvement.1,4 Of these patients, 90% to 100% have anterior and/or superior mediastinal and hilar adenopathy.1,4,19 This pattern is the most common manifestation of the nodular sclerosing subtype; peripheral and retroperitoneal adenopathy is more common with the mixed cellularity variant. Typically, multiple lymph node groups in the thorax are affected; this can produce a lobulated appearance on chest radiographs (Fig. 32-1A,B). This lymph node involvement is characteristically bilateral, with contiguous progression from one lymph node group to the next. Adenopathy of the internal mammary chains can occur, but involvement of these nodes in the absence of lymphadenopathy elsewhere in the chest has not been described.19 When the anterior/superior mediastinum is involved, invasion to the pleura, pericardium, or anterior chest wall may be seen. Furthermore, 15% to 40% of HL patients have pulmonary involvement, whereas such involvement is much less common in NHL.4,19 Pulmonary involvement can occur by direct extension from involved nodes, or it can take the form of pulmonary nodules that may be either ill- or well-defined. These nodules may be unilateral or bilateral, and they may cavitate. Other associated findings with intrathoracic HL include parenchymal consolidation, pleural effusions, and sternal erosions.1,8,19 Localized disease is usually treated with radiation therapy, whereas diffuse disease is treated with chemotherapy.
NHL is four times more common than HL and represents approximately 3% of all diagnosed cancers in the United States. It is the third most common childhood malignancy.4,8 NHL is more frequently fatal than HL.1 It encompasses a large variety of diseases of differing histology, prognosis, and treatment. Although there are many classification schemes for NHL, the most commonly used system in the United States is the Working Formulation for Clinical Usage devised by the National Cancer Institute.1,4 Under this scheme NHL is categorized into three grades (low, intermediate,
and high) based on histology and prognosis.1,20 Patients with low grades usually present with widespread disease, whereas patients with intermediate and high grades usually present with extranodal disease.1
and high) based on histology and prognosis.1,20 Patients with low grades usually present with widespread disease, whereas patients with intermediate and high grades usually present with extranodal disease.1
At present there is no specific staging system for NHL. As a result, the Ann Arbor system is commonly used. Staging is less important in NHL for developing treatment plans, because about 90% of patients at initial presentation have advanced disease of differing stages and are treated similarly.20
Although a definite cause for NHL has not been identified, there are several predisposing conditions that increase a person’s risk. Patients with human immunodeficiency virus infection, organ transplants requiring immunosuppression, or collagen vascular diseases are all at increased risk for NHL.4,8 This risk can be as high as 40 to 100 times the risk of a normal, healthy person. Also, a secondary NHL (usually large-cell lymphoma) develops in a percentage of patients with treated HL.20
Although NHL can affect persons of any age, the median age at the time of initial diagnosis is 55 years.8 At presentation, 40% to 43% of patients have intrathoracic disease, with approximately 87% of these patients having mediastinal and hilar lymphadenopathy.1,3 Although the middle mediastinum is most frequently involved, the posterior mediastinum and cardiophrenic angles can also be affected. Usually, however, the latter two regions are affected when involvement of a single lymph node is the only manifestation of intrathoracic disease.19 In contrast to HL, NHL tends to appear as a single large, conglomerate nodal mass rather than as individual enlarged nodes. Other common nodal sites of involvement include the lung parenchyma, pleura, and pericardium.8 In general, lower grades are treated with radiation therapy and higher grades are treated with chemotherapy.
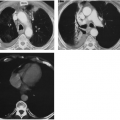
Radiographically, CT is the imaging study of choice for diagnosis, staging, and follow-up of patients with lymphoma (Fig. 32-1C). It can help identify tumor not suspected on plain films, often altering treatment plans.8 CT offers the advantage of better localization and characterization of the
disease process. Furthermore, it can demonstrate compression and involvement of adjacent structures in the mediastinum better than plain films can. Calcification is also more readily apparent with CT. This is important because calcification is uncommon in untreated lymphoma, and its presence should suggest a different diagnosis. However, irregular, eggshell calcifications have been reported in patients with untreated malignant lymphoma.1 On CT, nodes appear enlarged and demonstrate homogeneous attenuation similar to muscle with little enhancement. Central areas of lower attenuation, indicating necrosis, are present in 21% to 50% of patients with newly diagnosed HL.19 These necrotic nodes, however, have no prognostic significance.3,19 CT is commonly used to monitor the response to treatment.
disease process. Furthermore, it can demonstrate compression and involvement of adjacent structures in the mediastinum better than plain films can. Calcification is also more readily apparent with CT. This is important because calcification is uncommon in untreated lymphoma, and its presence should suggest a different diagnosis. However, irregular, eggshell calcifications have been reported in patients with untreated malignant lymphoma.1 On CT, nodes appear enlarged and demonstrate homogeneous attenuation similar to muscle with little enhancement. Central areas of lower attenuation, indicating necrosis, are present in 21% to 50% of patients with newly diagnosed HL.19 These necrotic nodes, however, have no prognostic significance.3,19 CT is commonly used to monitor the response to treatment.
Although CT and MRI can both be used to monitor response to therapy, MRI has certain advantages. MRI defines invasion and/or compression of vascular and cardiac structures better than CT without the use of intravenous contrast media. This is a definite advantage in patients with poor renal function or other contraindications to the use of iodinated contrast agents. On T1-weighted images, lymphomatous tissue has a signal intensity equal to or greater than that of muscle but less than that of fat. This signal is usually homogenous, but in larger masses heterogeneous signal is common. On T2-weighted images, signal intensity is greater than that of muscle. These findings are independent of the grade of the tumor.8 It is important to note, however, that residual disease after treatment usually demonstrates a higher T2 signal than scar or benign fibrous tissue. This is helpful because MRI can help differentiate benign tissue from residual tumor, sometimes obviating the need for further therapy.1,3,8
Germ Cell Tumors
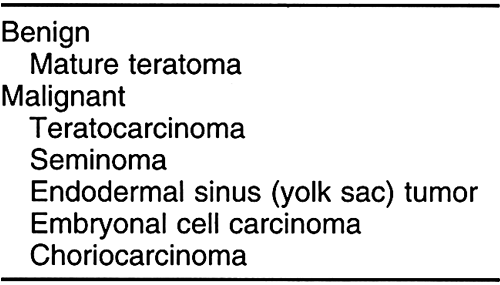
Germ cell tumors are classically divided into seminomatous neoplasms (seminoma) and nonseminomatous neoplasms (teratoma, embryonal cell carcinoma, endodermal sinus tumor or yolk sac tumor, choriocarcinoma). They account for approximately 1% to 3.5% of tumors of the mediastinum. Although most are found in the anterior mediastinum, approximately 10% are in the posterior mediastinum.3,4 These tumors begin during embryologic development when collections of primitive germ cells arrest in the anterior compartment on their journey to the gonads.4,11 Because they are histologically indistinguishable from gonadal germ cell tumors, the diagnosis of a primary mediastinal germ cell neoplasm requires exclusion of a primary gonadal tumor with mediastinal metastases.3
Patients are usually between the ages of 20 and 40 years, with an equal sex distribution. Benign tumors commonly affect women, whereas malignant tumors commonly develop in men (Table 32-3).8,19 Usually, patients with the benign types have normal levels of β-human chorionic gonadotropin (HCG) and α-fetoprotein, whereas malignant tumors may secrete high levels of either or both. These chemical markers can be used not only to diagnose the disease but also to monitor the effectiveness of therapy.1 Clinically, these tumors have similar signs and symptoms related to local compression and/or invasion of adjacent structures.1 Traditionally, CT has played a major role in the workup of mediastinal germ cell tumors, and MRI has been utilized to a very limited extent.
Seminomatous Neoplasms
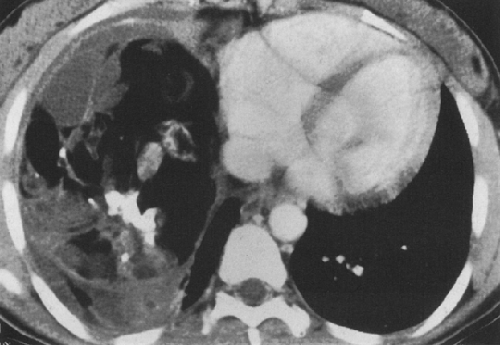
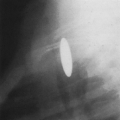
The seminomatous neoplasms (seminomas) are the second most common germ cell tumors, constituting 30% to 50% of germ cell neoplasms. They are the most common primary malignant germ cell tumors.3,4,8 These neoplasms are considered less aggressive than the nonseminomatous tumors. They secrete low levels of HCG and are commonly seen in young men.3 At CT, these neoplasms appear as large masses with sharply demarcated borders. They often have homogenous attenuation but may have interspersed areas of low attenuation, indicating hemorrhage and necrosis (Fig. 32-2). Chest wall invasion and calcification is uncommon, and these tumors often extend to the left of midline.1,4,19
They are usually treated with radiation alone, which has an 80% cure rate. Recently, cisplatin chemotherapy has proved to be an alternative treatment option for mediastinal seminomas. After treatment there can be a residual mass. If this mass is less than 3 cm in size, there is a low probability that it harbors residual malignant cells. As a result, unless the residual mass is noted to enlarge over time, it can be monitored closely without the need for surgical removal.8
Nonseminomatous Neoplasms
The nonseminomatous mediastinal neoplasms are more aggressive than the seminomatous tumors and secrete high levels of α-fetoprotein (80%) and/or HCG (30%). The teratomas are the most common nonseminomatous tumors and the most common mediastinal germ cell neoplasms. They can be classified into benign and malignant types. The benign varieties are composed of mature tissue elements from more than one embryonic germ cell layer, whereas the malignant varieties are composed of primitive, immature elements from these same layers. The benign variant has been termed a mature teratoma; it is classified histologically as an epidermoid, dermoid, or teratoma depending on the embryonic
germ cell layers that are present (Table 32-4). The malignant variant is called a teratocarcinoma; it can contain elements of seminoma, embryonal cell carcinoma, endodermal sinus tumor, choriocarcinoma, and sarcoma.1,4
germ cell layers that are present (Table 32-4). The malignant variant is called a teratocarcinoma; it can contain elements of seminoma, embryonal cell carcinoma, endodermal sinus tumor, choriocarcinoma, and sarcoma.1,4
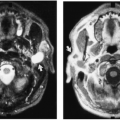
The benign or mature teratoma can be found at any age but is particularly common in young women. The benign teratomas are asymptomatic and usually are discovered incidentally on plain-film chest radiographs or CT scans. On radiographs, they appear as large, well-demarcated, rounded masses in the anterior mediastinum. These tumors are located in front of the roots of the aorta and main pulmonary artery and project mainly to one side of the midline.1,19 Calcification, ossification, or even teeth may be visible on radiographs. On CT, they usually appear as large, cystic masses that may contain elements from the different embryonic germ cell layers (Fig. 32-3). When ectodermal derivatives such as skin, sebaceous material, hair, and calcification predominate, they are known as benign cystic teratomas or dermoid cysts. They often have a thick, encapsulated wall that may enhance and may contain curvilinear calcification.1,19 Approximately 50% contain fat or material with attenuation like that of fat.19 Rarely, the cyst ruptures into the pericardium or pleura, in which case a fat–fluid level may be present. The presence of a fat–fluid level or fat within the mass makes the diagnosis almost certain.1
The malignant teratomas, or teratocarcinomas, are also found in young adults, but they are much more common in men. The great majority are symptomatic. The plain-film findings are similar to those of benign teratomas except that the mass is often more lobulated in outline, rarely has calcification, and never has fat density.1 The malignant forms grow rapidly and frequently metastasize to the lungs, bones, or pleura. At CT, the typical mass has an irregular border with a thick capsule that enhances with intravenous contrast. The adjacent mediastinal fat planes usually are obliterated, and extensive local invasion is common. These tumors tend to
be less cystic and have a more homogenous appearance, with interspersed areas of enhancement and low attenuation indicating hemorrhage and necrosis.
be less cystic and have a more homogenous appearance, with interspersed areas of enhancement and low attenuation indicating hemorrhage and necrosis.
Lesions of the Thymus
The normal thymus lies in a retrosternal location behind the manubrium. It is commonly seen anterior to the proximal ascending aorta and distal superior vena cava. In the newborn it usually is larger than the heart; in older children it may often extend inferiorly to occupy the space anterior to the heart.8,19 The size of the normal thymus is largest between 12 and 19 years of age.19
The shape of a normal thymus is variable and has been described as resembling an arrowhead in 62% of patients, bilobar in 32%, and consisting of only one lobe in 6% of patients.2,19 It typically has an approximately symmetrical shape, with the left lobe usually larger than the right lobe.1 These lobes commonly connect in the midline, each lobe being surrounded by a fibrous capsule.19
On chest radiographs, the diagnosis of a normal thymus may be suggested by a change in size and shape of the anterior mediastinal mass on inspiration and expiration. On CT the normal thymus conforms to the space anterior to the great vessels, and its attenuation is in the range of +30 HU.19 In addition, the normal thymus undergoes fatty infiltration with age.8 CT typically allows detection of the normal thymus in 83% of subjects younger than 50 years of age and 17% of subjects older than 50 years of age.19 On MRI, normal thymic tissue has a homogenous signal similar to that of muscle on T1-weighted images and a signal intensity greater than muscle on T2-weighted spin-echo sequences.
Thymic Hyperplasia
Thymic hyperplasia is the most common anterior mediastinal mass in the pediatric age group up to puberty. Histologically, there is hyperplasia of the germinal centers. This lesion is often seen in association with hyperthyroidism, myasthenia gravis, acromegaly, or Addison’s disease.4,19
On the other hand, a form of thymic hyperplasia termed rebound hyperplasia occurs in children and young adults who are recovering from severe illness such as burns, after treatment for Cushing’s syndrome, or after chemotherapy. The thymus may regrow more than 50% above normal transiently, and steroid treatment has been shown to reduce the size of this hyperplastic gland.4,19
At CT there is diffuse, symmetrical enlargement of the gland. The gland conforms to the shape of adjacent structures, and its attenuation remains equal to or slightly greater than that of the surrounding chest wall musculature.1,19 According to Stark and Bradley,18 there appears to be no specific MRI signal pattern for thymic hyperplasia.
Thymic Cyst
Thymic cysts represent 1% to 2% of all mediastinal masses and are usually encountered in children. They are usually simple cysts (unilocular or multilocular); they can be congenital (persistence of thymopharyngeal duct), inflammatory, neoplastic, or postoperative in origin.1,4,8,19 Thymic cysts may also be found within thymomas, thymic germ cell tumors, and in patients who have been treated for Hodgkin’s disease with mediastinal radiation or chemotherapy.1,4,19 They are commonly asymptomatic, but some patients may experience pleuritic chest pain if hemorrhage into the cyst occurs.4,19
On plain films, thymic cysts are difficult to differentiate from other nonlobulated thymic masses. At CT, thymic cysts have the appearance of other fluid-filled cysts with water attenuation. If hemorrhage occurs, attenuation may be increased and calcification in the wall of the cyst may be seen.1,19 On MRI, thymic cysts commonly have low signal on T1-weighted images and homogenous high signal on T2-weighted images, characteristic of fluid. If hemorrhage into the cyst occurs, the thymic mass may demonstrate increased signal intensity on both T1- and T2-weighted images.1,8,18
Thymolipoma
Thymolipomas are rare tumors that occur in patients from 3 to 60 years of age. These masses contain fat and normal thymic tissue and can grow to a very large size before becoming symptomatic (50% of patients have chest pain, cough, or dyspnea).1,4,8 Because of their high fat content, thymolipomas usually mold to the adjacent mediastinal
structures and diaphragm.1,4 On chest plain films they may be mistaken for cardiomegaly or lung collapse.1 CT demonstrates the fatty nature of the mass along with interspersed residual thymic tissue. On MRI, the fatty mass shows a signal intensity that parallels that of subcutaneous fat on all pulse sequences.1,8,18
structures and diaphragm.1,4 On chest plain films they may be mistaken for cardiomegaly or lung collapse.1 CT demonstrates the fatty nature of the mass along with interspersed residual thymic tissue. On MRI, the fatty mass shows a signal intensity that parallels that of subcutaneous fat on all pulse sequences.1,8,18
Thymoma
Thymomas are the most common primary neoplasms of the anterior mediastinum.4,8,18,19 They arise from both the epithelial and the lymphocytic components of the thymus gland. Traditionally, thymomas have been classified pathologically into four types: encapsulated thymoma (intact fibrous capsule), locally invasive thymoma, metastasizing thymoma (benign cytologic appearance with pleural and pulmonary parenchymal seeding), and thymic carcinoma (frankly malignant histology and worse prognosis than other thymomas).1,4,8,19 Approximately 30% to 35% of thymomas are malignant, as defined by invasion into adjacent mediastinal fat and fascia.1,4,19
These tumors commonly occur in patients older than 40 years of age, with 70% occurring in the fifth or sixth decade.1,4,8 They are unusual before the age of 20 years and are equally common in male and female patients.1,19 Thymomas are classically asymptomatic. When symptoms do occur, they may be a result of local mediastinal compression, or they may be related to several associated parathymic syndromes. For example, 30% of patients with thymoma have myasthenia gravis, and 10% to 15% of those with myasthenia gravis have thymoma. Other hematologic disorders such as red blood cell aplasia and hypogammaglobulinemia have been reported.1,4,8,19
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree