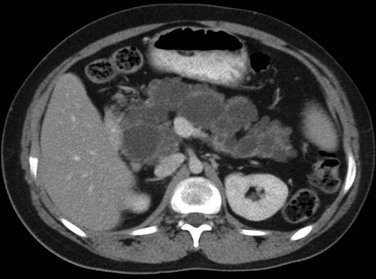
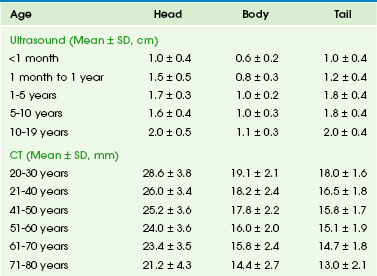
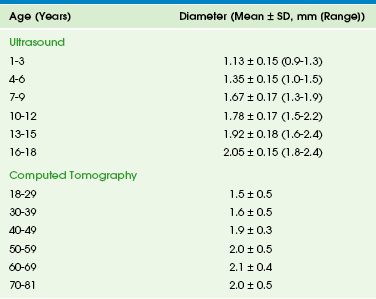
Chapter 96 Developmental deviation from this embryologic pattern can give rise to variants. The usual ductal configuration is most commonly bifid, formed by the ducts of Wirsung and Santorini (60% of cases). Less common configurations include a rudimentary duct of Santorini (30%); a dominant duct of Santorini (1%); and ansa pancreatica, in which the duct of Santorini curves as it courses to the duct of Wirsung. Ductal narrowing can be seen at the site of fusion of the dorsal and ventral ducts. The absence of proximal dilation allows differentiation of this normal variant from a true stricture. Duodenal obstruction, pancreatobiliary maljunction pancreatitis, and biliary cysts occur secondary to developmental variants. Pancreatobiliary maljunction is associated with congenital common bile duct webs.1 The pancreas grows substantially in the first year of life, and growth slows from year 1 through 18. The gland is relatively larger in children than in adults, and the overall ratio of gland size to patient body size decreases with age (Table 96-1). The pancreatic head is more prominent in children compared with the body and tail. The diameter of the pancreatic duct also varies with age (Table 96-2). Enlarged ducts (>1.5 mm at 1 to 6 years, >1.9 mm at 7 to 9 years, and >2.2 mm at 13 to 18 years) are associated with pancreatitis. Table 96-1 Normal Sonographic and Computed Tomographic Dimensions of the Pancreas Modified from Heuck A, Maubach PA, Reiser M, et al. Age-related morphology of the normal pancreas on computed tomography. Gastrointest Radiol. 1987;12:18-22; and Siegel MJ, Martin KW, Worthington JL. Normal and abnormal pancreas in children: US studies. Radiology. 1987;165:15-18. Table 96-2 Normal Diameter of the Pancreatic Duct by Ultrasound and Computed Tomography Modified from Siegel MJ, Martin KW, Worthington JL. Normal and abnormal pancreas in children: US studies. Radiology. 1987;165:15-18; Heuck A, Maubach PA, Reiser M, et al: Age-related morphology of the normal pancreas on computed tomography. Gastrointest Radiol. 1987;12:18-22; Chao HC, Lin SJ, Kong MS, et al. Sonographic evaluation of the pancreatic duct in normal children and children with pancreatitis. J Ultrasound Med. 2000;19:757-763; and Glaser J, Hogemann B, Krummenerl T, et al. Sonographic imaging of the pancreatic duct. New diagnostic possibilities using secretin stimulation. Dig Dis Sci. 1987;32:1075-1081. The pancreas lies transversely in the retroperitoneum. It is divided into the head, body, and tail (Fig. 96-1). The head is to the right of midline, situated within the “C-loop” of the duodenum. At the junction of the inferior and left margins of the pancreatic head is an extension of the gland called the uncinate process. The anterior surface of the pancreatic head is in contact with the transverse colon, gastroduodenal artery, and loops of small intestine. The anterior surface of the uncinate process is in contact with the superior mesenteric artery and vein. The posterior surface of the head is adjacent to the inferior vena cava, common bile duct, renal veins, and the abdominal aorta. Figure 96-1 Normal pancreas in an 11-year-old boy. The pancreas itself is not seen on plain radiographs, although calcifications in patients with chronic pancreatitis or cystic fibrosis may be identified on abdominal radiographs (Fig. 96-2). In acute pancreatitis, dilated loops of bowel and fluid levels within the upper midabdomen may suggest localized ileus. A pancreatic mass may be sufficiently large to displace adjacent gas-filled portions of the gastrointestinal (GI) tract.2 Figure 96-2 Cystic fibrosis in a 9-year-old girl. Ultrasonography (US) is the primary screening tool to evaluate the pediatric pancreas.3 The pancreas is most easily seen if the stomach and duodenum are not distended with gas. Ingestion of water devoid of gas bubbles may improve visualization. The distal body and tail may also be imaged in the prone position using the left kidney as an acoustic window. Age-matched normal dimensions of the pancreas are given in Table 96-1.3 Pancreatic size is best measured at its body, but individual variation is sufficient to warrant caution when determining pancreatic size. Enlargement of the pancreas should be diagnosed when the anteroposterior dimension of the pancreatic body is greater than 1.5 cm.4 The normal duct may be seen as a single- or double-track echogenic line anterior to the junction of the splenic and mesenteric veins (see Fig. 96-1). The pancreas has a spectrum of echogenicity relative to that of the liver, but in most children, the pancreas is hypoechoic or nearly isoechoic with the liver. However, in neonates, particularly in premature infants, the pancreatic gland is more echogenic.5 US is also helpful for image-guided biopsy and in aligning radiotherapy.6 Computed tomography (CT) of the pancreas7 is indicated less frequently than US, but it is valuable in certain conditions, particularly pancreatitis, tumors, and pseudocysts with uncommon features. The pancreas is best visualized during bolus injection of intravenous (IV) contrast material, which readily identifies the adjacent vessels, and with meticulous administration of GI contrast to opacify the adjacent stomach and duodenum. The pancreas is hypodense compared with the liver, both with and without intravenous contrast. The contours of the pancreas are commonly smooth but may be slightly lobulated. Because the pancreas in children is oblique to the axial plane, multiple thin sections may be necessary for optimal visualization; in-plane reconstructions, particularly from axial volumetric data obtained with multidetector equipment, can image the pancreas in its own oblique plane. Imaging of the pancreatic head may be optimized by scanning the patient in the right lateral decubitus position soon after ingestion of GI contrast material. With this technique, the optimally opacified duodenal C-loop outlines the pancreatic head, and opacified proximal jejunal loops outline the remainder of the gland. CT is the best modality for assessing neoplasms, pancreatic trauma, and pancreatitis and its complications and for further evaluation of abnormalities on US. Thin collimation volumetric CT coupled with curved planar reformations produces quality imaging of pancreatic and peripancreatic tissues.8 Magnetic resonance imaging (MRI) of the pancreas9,10 is more difficult in children than in adults because of adjacent gas-filled loops of intestine and motion artifact from peristalsis and respiration.11 Nevertheless, MRI is a powerful tool for imaging pediatric developmental abnormalities. The pancreas normally has signal intensity equal to that of liver on T1- and T2-weighted spin echo images with midfield strength magnets. Pancreatic images produced with high-field strength magnets may have greater signal intensity than those of the liver. To some degree, signal varies with age. Although normal children do not have as much intrapancreatic macroscopic fat as adults, adolescents have more fat in the pancreatic septa than do preadolescent children, and the amount of intrapancreatic fat may be increased in children with cystic fibrosis. The value of MRI is enhanced by the use of breath-holding techniques (generally not possible in younger children), fat suppression, contrast enhancement, and respiratory gating. Because of its noninvasive nature, magnetic resonance cholangiopancreatography (MRCP)12 may be more useful than endoscopic retrograde cholangiopancreatography (ERCP)13,14 in children (Fig. 96-3). Reported sensitivity, specificity, and accuracy are 87%, 90%, and 89% respectively for stones; 100%, 98%, and 98% for cholangitis; 92%, 97%, and 96% for bile duct tumors; and 89%, 96%, and 95% for periampullary stenosis.15 MRCP is also useful in certain congenital abnormalities, such as pancreas divisum, and after pancreatic trauma to identify duct of Wirsung transections. Although intravenous CT cholangiography is superior to MRCP in delineating postoperative anatomy after choledochal cyst repair, MRCP is highly accurate (84%) in depicting the anastomotic site, intrahepatic biliary tree, and reconstructed bowel, and it clearly demonstrates pancreatobiliary maljunction, residual distal common bile duct, common channel,16 and pancreatic duct.17 Similarly, MRCP accurately depicts the postoperative anatomy and complications after orthotopic liver transplantation.18 A normal MRCP may obviate the need for ERCP or percutaneous transhepatic cholangiography, and abnormalities visualized with MRCP can direct the modality and route for further intervention. An overview of MRCP pitfalls has been provided by Van Hoe and colleagues.19 Figure 96-3 Endoscopic retrograde cholangiopancreatography (ERCP) and magnetic resonance cholangiopancreatography (MRCP) in a 9-year-old with recurrent pancreatitis and stones. Secretin stimulation with MRCP further enhances the imaging information obtained, because it gives additional, valuable, functional and anatomic information about the pancreatic duct and pancreatic excretory capacity. Secretin-enhanced MRCP has been described in detail in recent years20–23 and has been found useful for detection and diagnosis of a variety of congenital, inflammatory, and neoplastic pancreatic conditions.24 Secretin causes temporary dilation of pancreatic ducts, principally by increasing pancreatic exocrine secretions, thus it allows better visualization of the ducts during MRCP. Congenital Pancreatic Abnormalities25,26 Overview: Pancreas divisum occurs when the dorsal and ventral ducts fail to fuse, although the pancreas is otherwise anatomically normal. This anomaly has been described in 4% to 14% of the population, depending on the method of evaluation. Clinical Presentation: In such cases, the accessory ampulla drains the major portion of the gland. It has been suggested that the incidence of pancreatitis is higher, although recent literature refutes this. Imaging: CT in children with pancreas divisum and pancreatitis demonstrates enlargement of both ducts, in addition to the characteristic findings of pancreatitis. Further enlargement of the ducts can be provoked with secretin stimulation.27,28 Increased thickness of the pancreatic head has also been described.29 Zeman and colleagues30 reported that thin-section CT demonstrated the unfused ducts in 5 of 12 patients (Fig. 96-4), and two distinct pancreatic moieties separated by a fat cleft was seen in 4 patients. Pancreas divisum may be associated with minor papilla adenoma beyond the childhood years. Figure 96-4 Pancreas divisum. Overview: Congenital short pancreas, also known as agenesis of the dorsal pancreatic anlage,32 occurs when the portion of the pancreas derived from the dorsal embryonic bud is absent, and only the smaller portion, derived from the ventral anlage, is present. Thus, the pancreatic neck, body, and tail are absent. This anomaly has been described in patients with polysplenia syndrome, or it may be a sporadic finding. Clinical Presentation: Variable symptoms of abdominal pain and epigastric discomfort and diabetes may be present.33 Imaging: Only a globular pancreatic head can be identified on CT (Fig. 96-5). Size is variable, and some patients show an enlarged or prominent pancreatic head, whereas others may show a normal sized or even mildly atrophic and small pancreatic head. The diagnosis of agenesis of the dorsal pancreas is inconclusive without demonstration of the absence of the dorsal pancreatic duct, either with MRCP or ERCP. Patients with this abnormality have an increased risk of developing diabetes mellitus34 because of the paucity of islet cells, most of which are located in the distal pancreas. This condition may also be associated in later life with pancreatic tumors35 such as intraductal papillary mucinous neoplasms. Overview: Ectopic pancreatic tissue36 is an aberrant rest of normal pancreatic tissue remote from the pancreatic body that occurs in 1% to 13% of the population. The vast majority of pancreatic rests (about 70%) are located in the stomach,37 duodenum, and jejunum, but they can occur elsewhere, such as omphaloenteric duct rest.38 An association with Beckwith-Weidemann syndrome has been reported.39 Imaging: Besides the findings described above, a noncommunicating gastric duplication cyst has been described that contained ectopic pancreatic ducts and islets without acini.36,40 Overview: Several theories of embryonic dysgenesis have been proposed for annular pancreas, but most suggest some form of rotational anomaly of the ventral bud, which may be bifid. The pancreatic annulus, or the portion surrounding the duodenum, frequently has a separate duct entering the duodenum opposite the ampulla of Vater. Duodenal contents may reflux through this duct into the annulus. Affected patients may have associated duodenal stenosis or atresia and may come to medical attention with duodenal obstruction in infancy. Many other associated abnormalities have been described, the most common being intestinal malrotation, tracheoesophageal fistula, anal atresia, and cardiac abnormalities. Clinical Presentation: Annular pancreas is frequently diagnosed in infancy because of associated duodenal obstruction. However, in approximately half the cases, the diagnosis is made beyond infancy (Fig. 96-6). The associated abnormalities described above are most common in patients who also have trisomy 21. Annular pancreas has also been described in de Lange syndrome, with heterotaxy, and as a cause of extrahepatic biliary obstruction.41 Pancreatitis that solely affects the annulus of an annular pancreas has been reported in adults.14 Figure 96-6 Annular pancreas. Imaging: MRI has advantages over CT in the diagnosis of annular pancreas, because with MRI it is easier to detect and characterize the tissue surrounding the duodenum as pancreatic. Diagnosis by US has also been described.42 ERCP and MRCP are used to investigate ductal anatomy. Coincidence of congenital short and annular pancreas with gallbladder agenesis and splenic malrotation is rare.43 Overview: Congenital cysts of the pancreas are rare and are often confused with choledochal,45 omental, or mesenteric cysts (e-Fig. 96-7). Single congenital pancreatic cysts are very rare and occur predominantly in females.46 e-Figure 96-7 Congenital pancreatic cyst. Clinical Presentation: Congential pancreatic cysts are usually asymptomatic, but when symptoms do occur, they are related to mass effect and compression of adjacent structures. Imaging: Congenital cysts are anechoic by US; they are usually unilocular, located in the pancreatic tail, and range in size from microscopic to 5 cm.47 Rarely, they may communicate with the ductal system. In contrast to single congenital pancreatic cysts, multiple congenital cysts may be associated with a polycystic disorder such as von Hippel–Lindau disease.48 Juxtapancreatic GI duplication cysts occur as abnormalities of the developing foregut and therefore usually have an alimentary tract epithelial lining. Most of these cysts arise from the stomach or duodenum but may rarely be sequestered within the pancreas.49 Overview: Cystic fibrosis (CF) leads to exocrine pancreatic insufficiency in 80% of affected patients. The pancreatic ductules contain goblet cells that produce abnormally thick mucus, leading to obstruction of enzyme egress pathways and associated pancreatic changes. Clinical Presentation: Patients have pancreatic insufficiency, mainly exocrine, with failure to thrive, abdominal distension, steatorrhea, and occasional rectal prolapse. Patients with pancreatic cystosis (see below) are usually asymptomatic. Imaging: In young patients with cystic fibrosis (CF), US50 shows a normal pancreas or pancreatic enlargement, but chronic obstruction ultimately results in shrinkage of the gland with fatty infiltration and fibrosis. On US, these histopathologic changes are visualized as increased echogenicity of the gland. CT shows a shrunken pancreas with reduced attenuation secondary to fatty infiltration. Fibrosis without fatty infiltration is found infrequently. Unenhanced scans may show pancreatic calcifications, ductal dilation, and pancreatic cysts (Fig. 96-8).51 MRI findings are variable but can accurately depict the changes of fatty infiltration, fibrosis, and atrophy.52–54 Figure 96-8 Cystic fibrosis (CF) with diffuse fatty replacement of the pancreas. Cystic transformation of the pancreas, or pancreatic cystosis, in children and young adults with CF has been described.55,56 This is an unusual form of pancreatic involvement with CF, in that the pancreas is replaced by macrocysts that are rarely more than 1 cm in diameter. This can be imaged with US, CT (Fig. 96-9), and MRI. These are true, epithelium-lined cysts that result from the accumulation of inspissated mucus, produced as a result of residual exocrine secretory function in the acinar cells, proximal to ducts obstructed from inflammation. Overview and Clinical Presentation: Shwachman-Diamond syndrome is an autosomal-recessive disorder that results in short stature, exocrine pancreatic insufficiency, metaphyseal chondrodysplasia, and bone marrow dysfunction. Imaging: US and CT evaluations of the pancreas demonstrate fatty replacement (pancreatic lipomatosis)57 as described earlier in patients with CF (e-Fig. 96-10). Other causes of pancreatic lipomatosis include chronic pancreatitis, prolonged steroid use, obesity, Cushing syndrome, hemochromatosis, obstruction of the duct of Wirsung, and Johanson-Blizzard syndrome. e-Figure 96-10 Shwachman-Diamond syndrome in an 8-year-old boy with anemia. Overview and Clinical Presentation: von Hippel–Lindau disease (VHL) is an autosomal-dominant disorder characterized by hemangioblastoma in multiple organs, especially the retina and central nervous system; skin lesions and cysts of numerous organs, including the pancreas. Pancreatic involvement is seen in approximately 21% of patients.58 Up to 75% of these multiple pancreatic lesions (e-Fig. 96-11) likely originate from progenitor cells, not mature endocrine cells as previously thought.59 e-Figure 96-11 von Hippel–Lindau disease in a young woman. Imaging: VHL-associated cysts are typically anechoic on US and have reduced attenuation on CT compared with the surrounding pancreatic tissue. Multiple cysts—as well as serous and mucinous cystadenomas of the pancreas, carcinoma, adenocarcinoma, and islet cell tumors—are also associated with VHL.60 Adenocarcinoma occurs in affected adults, and pancreatic calcifications can be seen on unenhanced CT scans. Overview: Autosomal dominant polycystic disease is a hereditary disorder with 100% penetrance but variable expressivity. Clinical Presentation: Symptoms of large pancreatic cysts include abdominal pain, jaundice, and fever. Imaging: Renal cysts are the dominant feature, but cysts may also be found in the liver, spleen, adrenal glands, and pancreas. Pancreatic cysts are present in about 10% of patients, and the gland is typically less involved than the kidneys or liver. Overview and Clinical Presentation: Beckwith-Wiedemann syndrome (BWS) is a relatively frequent overgrowth syndrome with an incidence estimated at 1 in 14,000 births. It is probably an autosomal-dominant disorder with variable transmission. The BWS gene has been identified on the short arm of chromosome 11 (11p15.5). The syndrome is characterized by visceromegaly, hemihypertrophy, and development of malignant tumors in 10% to 15% of affected patients; benign tumors are also found. Imaging: Cross-sectional imaging studies may show nonspecific pancreatic enlargement. Patients may develop pancreatoblastoma or nesidioblastoma, as discussed later under Pancreatic Neoplasms. Because of this risk, routine US screening is initiated at an early age.
The Pancreas
Embryology, Anatomy, and Physiology
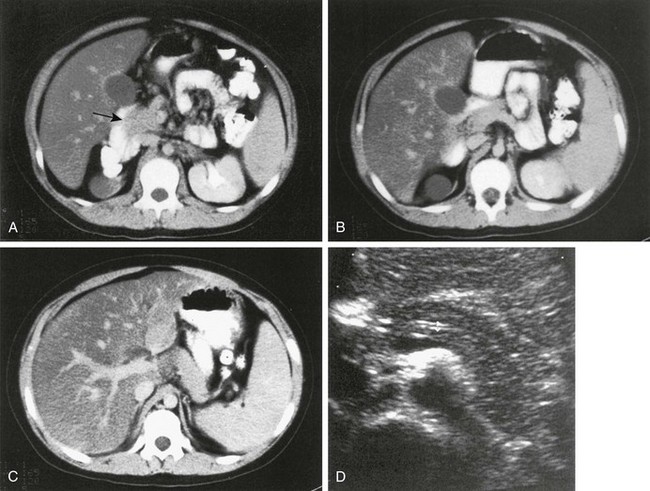
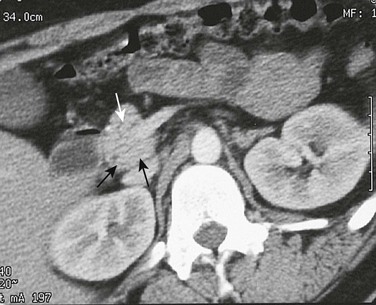
A to C, Computed tomography (CT) sections of the pancreas. The head of the pancreas (arrow in A) is slightly bulbous and distinct from the contrast-filled duodenal sweep. The body of the pancreas (B) is narrower than the head or tail and is seen anterior to the aorta, from which the superior mesenteric artery arises. The tail of the pancreas (C) is thicker in children than in adults and extends to the spleen. D, Transverse ultrasound shows the double track of a normal pancreatic duct.
Imaging the Pancreas in Children
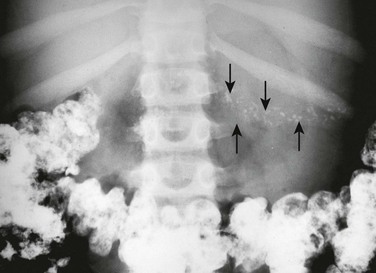
Multiple pancreatic calcifications (arrows) are seen on radiography.
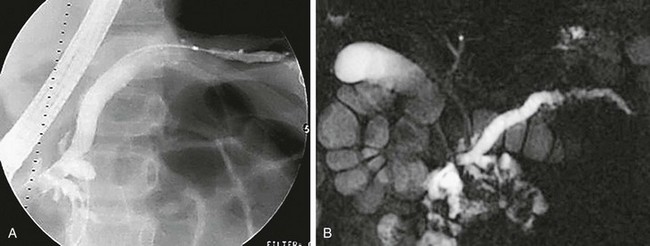
A, Anteroposterior image from ERCP shows abnormal dilation of the proximal pancreatic duct and narrowing, irregularity, beading, and an undulating caliber distally. B, Anterior MRCP image of the same patient similarly defines the abnormal caliber and irregularity of the pancreatic duct, consistent with recurrent pancreatitis. The common bile duct is normal. (Courtesy Dr. Kimberly Applegate, Indianapolis, IN.)
Congenital and Hereditary Pancreatic Abnormalities
Pancreas Divisum
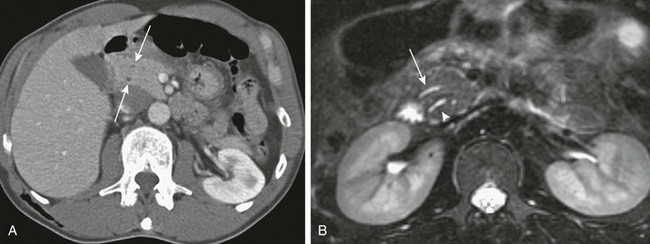
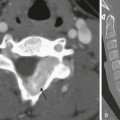
A, Axial contrast-enhanced computed tomography shows two pancreatic ducts (arrows). B, Axial fast spin-echo T2-weighted magnetic resonance image from a teenager with recurrent pancreatitis shows separate ducts of Santorini (arrow) and Wirsung (arrowhead) in the pancreatic head. (Courtesy Dr. Marilyn Siegel, St. Louis, MO.)
Congenital Short Pancreas
Ectopic Pancreas
Annular Pancreas
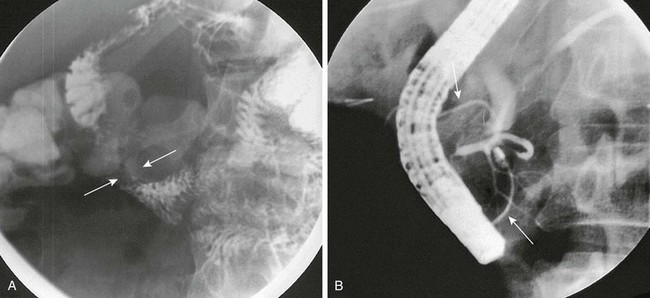
A, Oblique view during an upper gastrointestinal series shows extrinsic narrowing of the duodenal C-loop (arrows). B, Endoscopic retrograde cholangiopancreatography confirms the presence of small ducts (arrows) encircling the duodenum, consistent with an annular pancreas. (Courtesy Dr. George Taylor, Boston, MA.)
Congenital Pancreatic Cysts
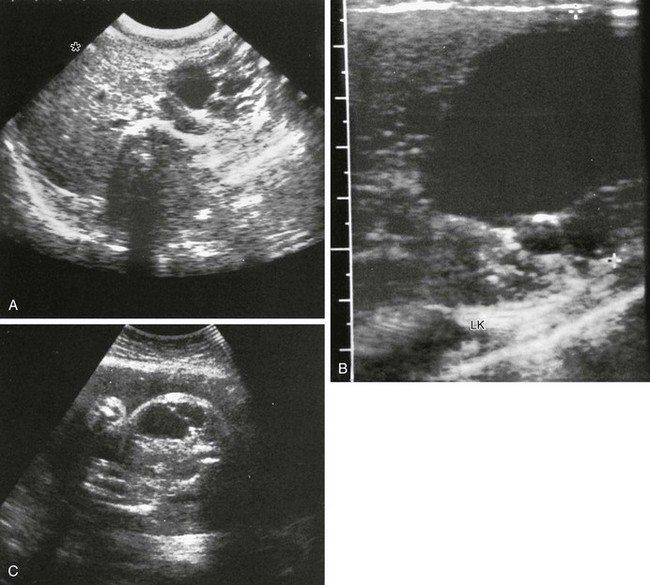
A-B, Transverse sonograms of the midabdomen in a newborn girl reveal a large anechoic mass. C, Antenatal ultrasound shows the cyst in the fetus. At surgery, a large congenital pancreatic cyst was found. LK, left kidney. (C, Courtesy Dr. D. McCallum, Palo Alto, CA; from Baker LL, Hartman GE, Northway WH Jr. Sonographic detection of congenital pancreatic cysts in the newborn: report of a case and review of the literature. Pediatr Radiol. 1990;20:488.)
Hereditary Systemic Conditions with Pancreatic Involvement
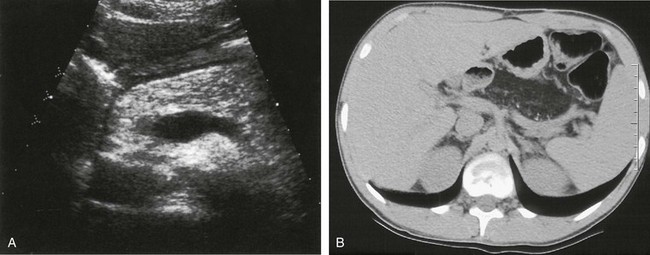
A, Markedly hyperechoic pancreas on US in an 18-year-old woman with CF and fatty infiltration of the pancreas. B, Axial noncontrast abdominal computed tomography shows diffuse fatty replacement of the pancreas with multiple tiny calcifications. (Courtesy Dr. Robert Kaufman, Memphis, TN.)
Shwachman-Diamond Syndrome
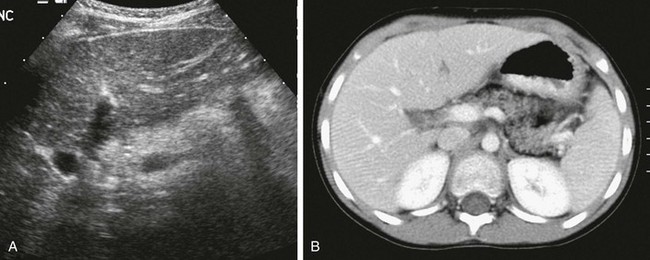
A, Transverse ultrasound through the pancreas shows increased echogenicity consistent with fatty infiltration. B, Corresponding axial contrast-enhanced computed tomography confirms partial fatty replacement of the pancreas. The patient also had coxa valga (not shown).
von Hippel–Lindau Disease
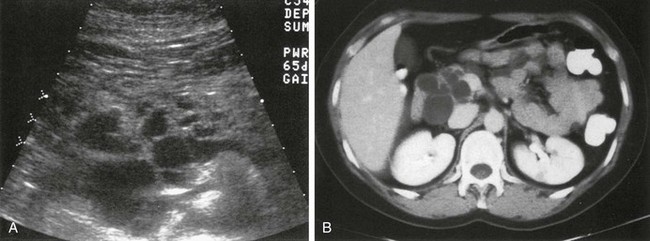
Multiple pancreatic cysts are well demonstrated on ultrasound (A) and computed tomography (B).
Autosomal-Dominant Polycystic Disease
Beckwith-Wiedemann Syndrome