Fig. 2.1
RT fields for a stage I seminoma
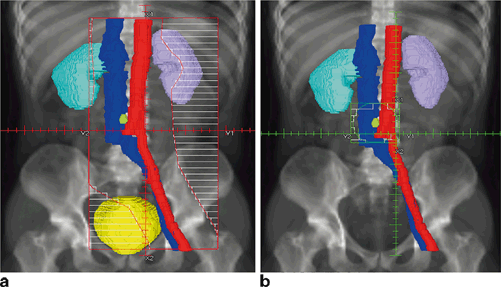
Fig. 2.2
a Initial RT fields for a stage IIA seminoma. Enlarged PA node in yellow-green. b Boost field for stage IIA seminoma
Urethral Cancer
Primary urethral cancers are extremely rare malignancies. SEER analysis from 1973 to 2002 identified an annual age-adjusted incidence rate of 4.3 per million US men and 1.5 per million US women [40]. A multimodal approach to treatment is commonly employed with a goal of organ preservation whenever possible; however, given the relative rarity of the disease and historical lack of treatment uniformity, the role of RT is not well described through randomized clinic trials.
RT has historically played a limited role in the treatment of male urethral cancers. Rabbani et al. identified 2065 men from the SEER database from 1988 to 2006 with primary urethral cancer. Of these patients, 78 % had urothelial carcinoma histology, 67 % presented with less than or equal to T1 disease and 61 % of patients were managed with simple surgical excision alone. Only 10 % of patients received radical resection and RT was utilized to only 10 % of patients as well. The SEER database did not distinguish between proximal vs distal tumors and so it is unclear how tumor site may have influenced treatment choice [41]. Outcomes regarding male urethral cancers treated with radiation are limited. In one of the larger retrospective series on the topic, Dalbagni et al., retrospectively reviewed 46 men with primary urethral carcinoma treated at Memorial Sloan Kettering Cancer Center (MSKCC) between 1958 and 1996. Forty patients received surgery alone and 6 received RT followed by salvage surgery. In this study, none of the RT patients responded to RT though the authors posit that this was due in large part to selection bias and higher T stage among RT patients [42]. Radical CRT for more advanced male urethral carcinoma has showed promise in at least one small, single-institution analysis. Eighteen men in this study with T2–4, N0–2 disease were treated on a protocol of 45–55 Gy, to a field encompassing the inguinal, external iliac lymph nodes and genitalia from the perineum to the upper sacrum using AP/PA technique with a boost of 12–15 Gy to the primary lesion. Radiation was given concurrently with mitomycin and 5-fluorouracil (5-FU). Results demonstrated that 83 % of patients had a complete response to treatment with 5-year overall and disease-specific outcomes of 60 and 83 %, respectively. Three of the nonresponders and four of the complete responders who recurred required salvage surgeries [43].
Radiation has a more established role in the treatment of female urethral cancers though outcomes remain poor irrespective of the choice of treatment modality. Several long-term retrospective series have analyzed the role of RT in female urethral cancers. Grigsby et al. published the results of 44 patients with urethral carcinoma, of whom 12 received RT with surgery (either pre- or postoperatively, dose range: 30–73.68 Gy, median 50.4 Gy) and 25 received EBRT and brachytherapy (EBRT doses 12–70 Gy, median 42.72 Gy; brachy doses 15–145 Gy, median 80 Gy). EBRT fields included bilateral groins in all women. The 5-year OS rate was 42 % and the 5-year cause-specific survival was 40 % with the aggressive treatment regimens resulting in high complication rates [44]. Similarly, Garden et al. reviewed the outcomes of 97 women treated for primary urethral carcinoma at MD Anderson Cancer Center. Of those, 86 received radiation after excision or biopsy, including 35 treated with combined EBRT and IB (EBRT doses 20–70 Gy, median 46 Gy, Brachy doses 20–70 Gy, median 30 Gy), 21 treated with EBRT only (40–71 Gy, median 61 Gy), and 30 with IB only (45–75 Gy, median 60 Gy). There was significant heterogeneity among treatment techniques and fields employed. The overall actuarial 5-, 10-, and 15-year survival rates for all 97 patients were 41, 31, and 22 %, respectively, and the type of treatment did not predict outcome [45]. Princess Margaret Hospital published results of 34 women with urethral carcinomas treated with radiation that was directed to the primary lesion in 15 patients vs the primary tumor and regional lymph nodes in 19 patients. Of these patients, 20 received combined EBRT and brachytherapy. The median dose to the primary tumor, accounting for the contributions of both EBRT and brachytherapy doses for all 34 patients, was 57 Gy (range 30–83 Gy). The 7-year actuarial overall and cause-specific survivals were 41 and 45 %, respectively, and brachytherapy reduced the risk of local recurrence by a factor of 4.2 [46].
Penile Cancer
Penile cancer is a rare GU malignancy in the USA with estimated 1600 new cases in 2014, accounting for less than 1 % of male malignancies [1]. Although uncommon in Europe and the USA, it represents a more significant cause of male cancer in the Indian subcontinent, Africa, and Latin America. The conventional treatment for early stage penile squamous cell carcinoma has been total or partial penectomy, which results in rates of local control in excess of 90 % [47]. In recent years, however, there has been a trend towards organ-sparing treatments including definitive EBRT and/or brachytherapy as a means to limit functional and psychosexual morbidity associated with penectomy. In the USA, however, use of RT for treatment of penile cancers remains limited. A recent SEER database analysis of 2427 men with penile cancer treated between 1988 and 2006 demonstrated 90.0 % received surgery alone, 2.2 % received EBRT alone, and 7.4 % received EBRT after surgery. One subject received brachytherapy alone and eight subjects received brachytherapy after surgery either with or without EBRT. Patients who received EBRT alone or in conjunction with surgery were more likely to have advanced T and N stages. The study authors posit that underutilization of RT for penile cancer is a function of referral bias, with patients presenting first to a dermatologist or urologist being offered specialty-specific therapy instead of referral to a radiation oncologist [47].
Despite the lack of widespread utilization, retrospective data have shown promising results for definitive RT for penile cancers. Ozsahin et al. published the results of a multicenter retrospective review of 60 patients with penile carcinoma. In total, 27 patients underwent surgery with or without adjuvant radiation vs 29 who underwent definitive EBRT alone. After biopsy, four patients refused RT. Of the patients receiving definitive EBRT, local control was obtained in 39 % and four patients who recurred underwent salvage surgery resulting in a penis preservation rate of 52 %. The 5-year and 10-year probability of surviving with an intact penis was 43 % and 26 %, respectively, and there was no significant survival difference between the patients treated with definitive RT and primary surgery (56 % vs 53 %; p = 0.16) [48]. A review of 67 men with T1–T3 penile cancers treated at two Canadian centers with penile-conserving primary brachytherapy revealed 10-year actuarial OS and cause-specific survival rates of 59 % and 83.6 %, respectively. Salvage penectomy was required for eight local failures and two cases of necrosis, for an actuarial penile preservation rate at 5 years of 88 % and 10 years of 67 % [49].
Although the role of adjuvant RT for penile cancer is not well defined in the literature, it appears to be most important in patients with positive pelvic lymph nodes. Franks et al. retrospectively analyzed the results of 23 men with pathologic N1–N3 penile cancer treated with adjuvant RT after local surgery and unilateral or bilateral groin dissection. The RT dose was 45 Gy in 20 fractions to the pelvis and bilateral groins delivered AP/PA. A 12-Gy boost in five fractions could be given if indicated. 3-year OS and locoregional relapse-free survival was 66 % and 56 %, respectively [50].
Summary
RT has a well-established and continually evolving role in the treatment of many of the most common and some of the rarest GU malignancies in the USA. Outcomes in many of these disease sites have sufficiently improved such that patients will live long enough to manifest not only the acute but also late-effects of treatment. There is an important duty on the part of all medical practitioners involved in the care of patients with GU malignancies to learn to appropriately prevent, diagnose and manage these treatment-related toxicities.
References
1.
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA (a cancer journal for clinicians). 2014;64(1):9–29.PubMed
2.
3.
Mohler JL, Kantoff PW, Armstrong AJ, Bahnson RR, Cohen M, D’Amico AV, et al. Prostate cancer, version 1.2014. J Natl Compr Canc Netw. 2013;11(12):1471–9.PubMed
4.
Moses KA, Paciorek AT, Penson DF, Carroll PR, Master VA. Impact of ethnicity on primary treatment choice and mortality in men with prostate cancer: data from CaPSURE. J Clin Oncol. 2010;28(6):1069–74.CrossRefPubMedCentralPubMed
5.
6.
Lawton CA, DeSilvio M, Roach M, 3rd, Uhl V, Kirsch R, Seider M, et al. An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: updated analysis of RTOG 94-13, with emphasis on unexpected hormone/radiation interactions. Int J Radiat Oncol Biol Phys. 2007;69(3):646–55.CrossRefPubMedCentralPubMed
7.
8.
Cooperberg MR, Lubeck DP, Meng MV, Mehta SS, Carroll PR. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol. 2004;22(11):2141–9.CrossRefPubMedCentralPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree