The Spleen
Tanya D. Nolan
|
OBJECTIVES
Describe the normal anatomy and function of the spleen.
Describe the normal vasculature of the spleen.
List the common causes of splenomegaly.
Demonstrate the scanning techniques used to image the spleen.
Identify the sonographic appearance and etiology of benign focal lesions of the spleen including splenic cyst, abscess, infarct, hematoma, and hemangioma.
Discuss the sonographic findings of lymphoma, leukemia, and metastases of the spleen.
Identify technically satisfactory and unsatisfactory sonographic examinations of the spleen.
KEY TERMS
abscess
accessory spleen
AIDS
angiosarcoma
asplenia
hamartoma
hemangioma
Hodgkin lymphoma
hypoplasia
leukemia
metabolic diseases
non-Hodgkin lymphoma
parasitic cyst
polysplenia
red pulp
sickle cell disease
splenic rupture
splenomegaly
wandering spleen
white pulp
GLOSSARY
erythrocyte red blood cell; contains hemoglobin and is responsible for transporting oxygen
erythropoiesis process of red blood cell production; occurs in the fetal spleen from the fifth to sixth month of fetal life after which the bone marrow assumes the function
hematocrit laboratory value of the percentage of blood volume made up of red blood cells; can be low in cases of anemia, blood loss, and leukemia
infarct tissue death caused by an interruption of blood supply
leukocyte white blood cell; main function is to protect against and fight infection in the body
leukocytosis elevated white blood cell count usually owing to infection
leukopenia decreased white blood cell count; can be a result of many factors including viral infection and leukemia
A thorough sonographic examination of the left upper quadrant (LUQ) includes the spleen. Because of its asymmetric shape and its location behind the ribs, the spleen can be difficult to orient, align, and elongate appropriately. Radionuclide imaging and computed tomography (CT) are often used to image the spleen; however, sonography is effective in characterizing splenic masses, evaluating splenic size and echotexture, identifying and characterizing palpated LUQ masses, monitoring the course of splenic trauma, and locating intraperitoneal blood collections.
EMBRYOLOGY AND NORMAL ANATOMY
During the fifth week of embryology, the spleen develops from the mesenchymal cells located between the layers of the dorsal mesentery. The spleen is not considered an endodermal derivative of the primitive gut although it does share an arterial supply with the foregut organs. The spleen moves from its original median position toward the LUQ as the stomach rotates and the mesogastrium develops.1
Eventually, splenic mesenchymal cells differentiate to form splenic pulp, connecting tissues, and the splenic capsule.
Eventually, splenic mesenchymal cells differentiate to form splenic pulp, connecting tissues, and the splenic capsule.
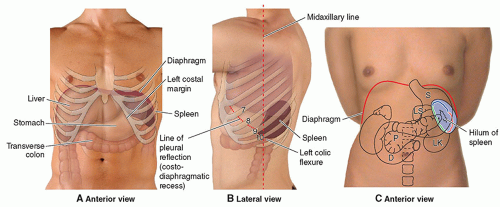 FIGURE 10-1 The spleen in relationship to surrounding organs and structures. D, duodenum; LK, left kidney; LS, lesser sac; P, pancreas; S, stomach. |
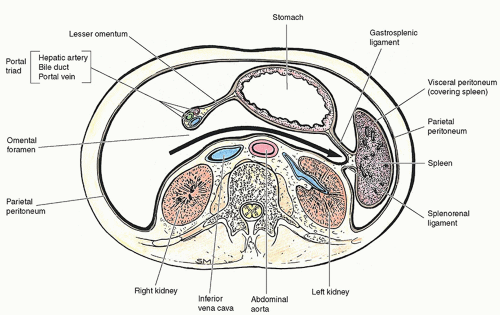
The spleen is an intraperitoneal, ovoid organ entirely covered by peritoneum except at a small bare area at the hilum through which the splenic artery, splenic vein, and efferent lymphatic vessels pass. The spleen is a delicate and vulnerable organ, and its long axis parallels the ninth to eleventh ribs2 (Fig. 10-1). The spleen is bordered anteriorly by the stomach; medially by the left kidney, splenic flexure of the colon, and pancreatic tail; and posteriorly by the diaphragm, pleura, left lung, and ribs1,3 (Fig. 10-2). When imaging the spleen, the neighboring organs and structures may create indentations and impressions on its visceral surface that may simulate masses.3 Therefore, knowledge of the normal variants of the spleen is essential in preventing a misdiagnosis.
Although the spleen has some mobility, the organ does not normally extend inferiorly beyond the left costal margin. As
a result, clinically palpating the spleen through the anterior lateral wall is difficult without significant enlargement of the organ.2 Moderate evidence suggests that palpation may be beneficial in supporting the diagnosis of splenomegaly, but this clinical examination cannot accurately rule out alternative conditions and must be correlated with diagnostic imaging.4
a result, clinically palpating the spleen through the anterior lateral wall is difficult without significant enlargement of the organ.2 Moderate evidence suggests that palpation may be beneficial in supporting the diagnosis of splenomegaly, but this clinical examination cannot accurately rule out alternative conditions and must be correlated with diagnostic imaging.4
At the hilus, the splenic arteries and veins are covered by the mesentery of the lienorenal ligament, which also houses the tail of the pancreas. The lienorenal and gastrosplenic ligaments attach the spleen to the left kidney and greater curvature of the stomach, respectively.2,3 The phrenicocolic ligament is not directly attached to the spleen; however, it supports the inferior end of the organ. The lienorenal, gastrosplenic, and phrenicocolic ligaments work together to stabilize the spleen and hold it loosely in position. Laxity of peritoneal attachments allows for hypermobility or wandering of the spleen.5
The average spleen measures 12 cm in length, less than 8 cm in anteroposterior dimension, and less than 4 cm in transverse dimension.6,7 In children, spleen length increases with age and is correlated with body parameters (height, weight, and body surface area). The formula used to determine spleen size in children is 5.7 + 0.31 × age (years). Infants who are 3 months or younger should have a spleen less than 6 cm in length. The adult splenic size is commonly compared to that of a person’s fist and weighs less than 150 g.8,9 Splenic size and weight vary based on age, gender, and nutritional status. The normal spleen is smaller in women, decreases in volume and size with advancing age, and increases in size during digestion.2,9 It has been reported that 3D sonographic visualization and measurement of the spleen volume have a moderate-to-high agreement when compared with CT volumetry.10
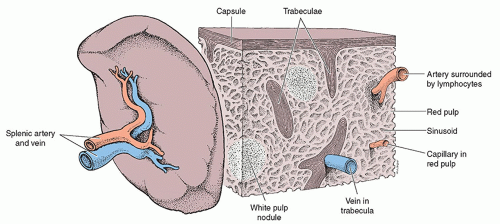
Overall, the spleen has the greatest amount of lymphoid tissue within the human body.1 A fibrous capsule composed of dense fibroelastic connective tissue surrounds the spleen and is thickened at the splenic hilum. Strands of connective tissue project from the splenic capsule and divide the spleen into several compartments. These communicating compartments are filled with splenic lymphoid tissue termed splenic pulp, which function to filter the peripheral blood2,8 (Fig. 10-3). The presence of white and red splenic pulp within the spleen make its texture soft and sponge-like. The white pulp is clustered around splenic arterioles and activates immune response when antigens and their antibodies are present within the blood.1,7 The red pulp consists of a network of blood-filled venous sinuses and reticular splenic cords, termed the Cords of Billroth. The red pulp is dedicated to seizing foreign particles and old, damaged, or mutated erythrocytes.1 Venous sinuses also act to store more than 300 mL of blood depending upon the systematic blood pressure. When the blood pressure drops, venous sinuses are constricted and may eject as much as 200 mL of blood into the venous circulation in an effort to restore blood volume.7,8
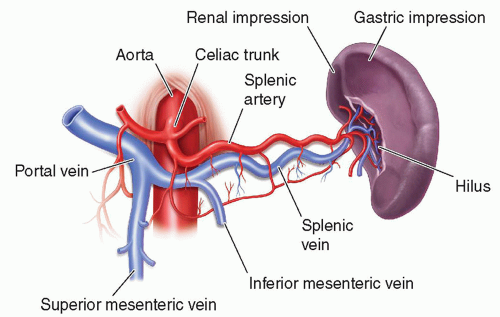
The highly vascular spleen receives its arterial blood from the splenic artery, a branch of the celiac axis (Fig. 10-4). This artery courses along the superior pancreatic border
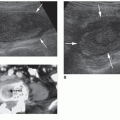
and divides into the superior and inferior terminal branches before entering the splenic hilum. Within the hilum, the splenic artery divides into six or more segmental branches before separating into several minor arterioles within the spleen.2,3,6 These arterioles may be visible as small echogenic lines passing through the splenic parenchyma.11 The intrasplenic arterial branches do not anastomose or communicate to create collateral flow. Without collateral flow, the spleen is at increased risk for infarction. Small branches of the segmental arteries provide blood to the white pulp before flowing into the venous sinusoids. This blood is then transported by pulp veins through splenic trabeculae to the splenic vein.6 Eventually, the splenic vein is joined by the inferior mesenteric vein and travels posterior to the tail and body of the pancreas. The splenic vein then unites with the superior mesenteric vein posterior to the neck of the pancreas to form the hepatic portal vein.2,3,6
and divides into the superior and inferior terminal branches before entering the splenic hilum. Within the hilum, the splenic artery divides into six or more segmental branches before separating into several minor arterioles within the spleen.2,3,6 These arterioles may be visible as small echogenic lines passing through the splenic parenchyma.11 The intrasplenic arterial branches do not anastomose or communicate to create collateral flow. Without collateral flow, the spleen is at increased risk for infarction. Small branches of the segmental arteries provide blood to the white pulp before flowing into the venous sinusoids. This blood is then transported by pulp veins through splenic trabeculae to the splenic vein.6 Eventually, the splenic vein is joined by the inferior mesenteric vein and travels posterior to the tail and body of the pancreas. The splenic vein then unites with the superior mesenteric vein posterior to the neck of the pancreas to form the hepatic portal vein.2,3,6
VARIANTS OF NORMAL
There are numerous congenital variations of the spleen.1 Several studies divide broad-spectrum splenic anomalies associated with heterotaxia syndrome into asplenia and polysplenia. Heterotaxy is related to a disruption in the normal embryologic development of left-right symmetry. This condition results in abnormal organ positions, situs, and/or arrangement.
Asplenia is the congenital absence of the spleen, also known as Ivemark syndrome.1,6 Splenic aplasia may be diagnosed in cases of congenital absence, surgical removal, or atrophy resulting from arterial or venous occlusions.12 Asplenia is associated with right-sided morphology of the heart and lungs. Often, the patient’s lungs are trilobed, and both main bronchi are located above the main pulmonary arteries.13 Other congenital malformations associated with asplenia include cardiovascular anomalies, situs ambiguous complexes, and visceral heterotaxia.6,14 The major causes for mortality and morbidity among patients with congenital asplenia are related to cardiac malformations.14
Splenic hypoplasia is characterized by a small pathologic spleen with reduced function resulting from abnormal development or parenchymal involution.14 Often, acquired hyposplenia is caused by sickle cell anemia. Some suggest that functional hyposplenism may be underdiagnosed as an immunodeficiency condition in children. Hyposplenic patients are at increased risk to develop solid tumors and vascular, autoimmune, and thrombolytic diseases. The major cause of mortality and morbidity among patients with hyposplenia is pneumococcal sepsis.1
Polysplenia is characterized by the presence of multiple smaller spleens, of similar size.1,13 In cases of polysplenia, there is a left-sided dominance in lung and cardiac morphology.6 Often, patients with polysplenia demonstrate bilobed lungs with the main bronchi found below the pulmonary arteries. Other associated anomalies include visceral heterotaxia, malrotations of the intestine, short pancreas, inferior vena cava (IVC) anomalies, cardiac defects, and biliary atresia.13
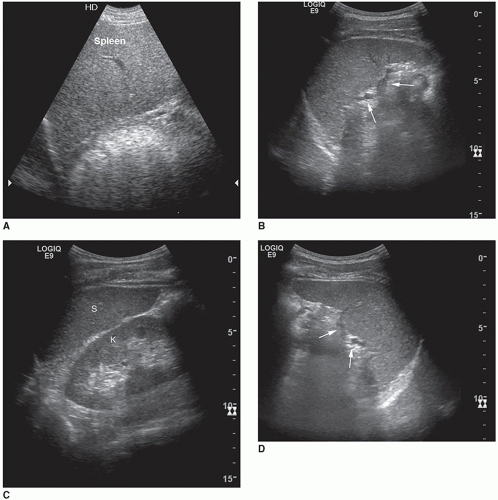
An accessory spleen is a common anatomic variant seen in approximately 10% to 30% of the population. Accessory (supernumerary) spleens occur when a portion of splenic tissue separates itself from the main body of the spleen and is found in an ectopic position.1,15 Most accessory spleens are small and measure approximately 2.0 cm.16 Approximately 75% of accessory spleens are found near the splenic hilum or gastrosplenic ligament (Fig. 10-5). The other 20% are located within the tail of the pancreas where they may be mistaken for hypervascular pancreatic tumors. Only 5% occur along the splenic artery and in the gastrosplenic, splenocolic, or gastrocolic ligament.1,15 Differentiating accessory spleens from pancreatic masses or from hilar lymph nodes may be difficult unless it is possible to trace their blood supply to the splenic artery. Sonographically, accessory spleens present as round, mildly echogenic, and homogeneous with posterior enhancement.17 Following splenectomy, an accessory spleen may assume the function and size of the removed organ. In cases of hematologic disorders, residual splenic tissue and accessory spleens present after laparoscopic splenectomy may lead to a relapse.1,18 Rarely, accessory spleens undergo
torsion or infarction clinically associated with acute LUQ pain. In most cases, accessory spleens are of no clinical consequence.6,17
torsion or infarction clinically associated with acute LUQ pain. In most cases, accessory spleens are of no clinical consequence.6,17
The wandering or ectopic spleen is a rare occurrence and is a spleen that migrates from its normal LUQ position to another location within the abdomen or pelvis. Wandering spleen is associated with a loss or a weakening of supporting ligaments.6,19 Developmental ligamental laxity occurs when there is an incomplete fusion of the dorsal mesentery with the posterior peritoneum or secondary to hormonal changes and maternal influences during pregnancy. With age, laxity may be related to splenomegaly, trauma, extreme weight loss, weak abdominal muscles, and gastric distension. Because the wandering spleen lacks its normal peritoneal attachments, this variant is associated with a high incidence of splenic torsion and infarction.1,20
Clinically, patients with a wandering spleen may present as asymptomatic or have varying degrees of abdominal pain. Sonographers should extensively examine the left side of the patient from the thorax to the pelvis in cases where the normal spleen is not imaged in its anatomic position. Contrast-enhanced CT is the imaging modality of choice in the diagnosis of wandering spleen. However, variable echo patterns in sonography combined with duplex Doppler and color flow imaging are sensitive in demonstrating a lack or absence of blood flow secondary to splenic artery torsion.19, 20 and 21 The preferred treatment for wandering spleen is splenopexy wherein a surgeon repositions the spleen in the LUQ to prevent torsion of the splenic vessels and preserve splenic function.21
PHYSIOLOGY
The spleen is an organ of mystery and perplexity for physiologists. Although the spleen is rarely the primary site of disease, it is often involved in inflammatory, hematopoietic, and metabolic disorders associated with immune and hematologic diseases. Functions of the spleen overlap those of other body organs, making it possible for a person to live without a spleen. However, studies completed on individuals with splenic absence have given some indication to the importance of its function. For example, patients who underwent splenectomy often suffered from leukocytosis, decreased circulating iron, decreased immune response, and an increased presence of circulating morphologically defective blood cells.8,9,22 Physiologically, the four major functions of the spleen include reserving, filtering, producing, and defending blood products.7,8 Within the spleen, the red pulp is dedicated to filtration, the white pulp is dedicated to adaptive immunity, and the perifollicular zone—located between red and white pulp—acts to connect both functions.23
Functions of the Spleen
Reservoir and Filter
The spleen acts as a reservoir for blood because a small volume of the blood entering the terminal capillaries of the spleen continues to circulate and enter highly distensible venous sinuses. This blood reservoir is crowded with red blood cells (RBCs) and platelets that may be used to provide a transfusion-type response when the body is stressed by hemorrhage.8 The majority of circulating blood, however, will pass through the hyperpermeable capillary walls into the red pulp for the purpose of filtration.7,8
Destruction of Red Blood Cells and Microorganisms
Approximately 5% of cardiac output is filtered every minute by the spleen.23 Within the red pulp, resident macrophages ingest and destroy unwanted debris; microorganisms; and old, damaged, or dead blood cells, particularly erythrocytes. Phagocytosed erythrocytes are catabolized, and the freed iron is stored in the macrophage cytoplasm or released back into the blood plasma.8,23,24 Ferritin, the iron protein complex, may be routed to the bone marrow and used to synthesize new hemoglobin whereas the broken down erythrocytes aid in the formation of bile pigments.
Defective cells, such as spherocytes, sickle cells, and thalassemic cells, are removed from circulation as they travel through the sinus walls. These cells lack the biconcave shape of normal RBCs, and the removal process is termed splenic culling. RBCs that contain an unwanted granule, or even a parasite, are not culled. Rather, these nuclear fragments or membrane inclusions are milked from the erythrocytes, and the cleansed RBCs are returned to normal circulation. This process is referred to as splenic pitting.23,25
Erythropoiesis
The spleen is responsible for erythropoiesis from approximately the fifth to the sixth months of fetal life. With age, the bone marrow assumes this primary function, but the spleen retains its capacity to produce RBCs throughout an adult’s life. The spleen’s hematopoietic functions can be regained if chronic anemia develops or bone marrow parenchyma is lost.8,24
Defense Against Disease
Lymphoid organs function as sites of proliferation, differentiation, or function of lymphocytes and mononuclear phagocytes.8 The white pulp, within the spleen, produces lymphocytes and plasma cells needed to form antibodies. There are many different types of lymphocytes including the T cells, B cells, and plasma cells.8,25 As a secondary defense, the spleen is able to phagocytose bacteria bypassed by the lymph nodes.
Laboratory Values
Normally, there are approximately 5,000 to 10,000 white blood cells (WBCs) per microliter of blood. Leukocytosis occurs when the leukocyte count is higher than normal.8,24 When a physiologic stressor is present, leukocytosis is a normal response and may indicate the presence of inflammation, infection, hemorrhage, carcinoma, and/or acute leukemia. In contrast, an abnormally low level of WBCs is referred to as leukopenia. A decrease in leukocyte counts below 1,000 per mm3 increases a patient’s risk for disease. Patients with counts below 500 per mm3 are in jeopardy for serious life-threatening infections. Leukopenia may result from radiation therapy, chemotherapeutic agents, systemic lupus erythematosus, vitamin B12 deficiency, Cortisol treatment, and anaphylactic shock. Leukopenia has also been
associated with hypersplenism, viral infections, leukemia, aplastic anemia, and diabetes mellitus.8,24
associated with hypersplenism, viral infections, leukemia, aplastic anemia, and diabetes mellitus.8,24
A laboratory hematocrit indicates the percentage of blood volume occupied by RBCs. The volume of RBCs circulating within the body is greatly influenced by the spleen’s sequestering and destructive function. Abnormal findings in the level of hematocrit result from altered erythropoiesis, anemias, hemorrhage, Hodgkin disease, and/or leukemia.8,24
Abnormal laboratory tests, history of infectious disease, LUQ pain, and a palpable enlarged spleen are all clinical manifestations and indications for a sonographic examination of the spleen.1,7
NORMAL SONOGRAPHIC APPEARANCE AND TECHNIQUE
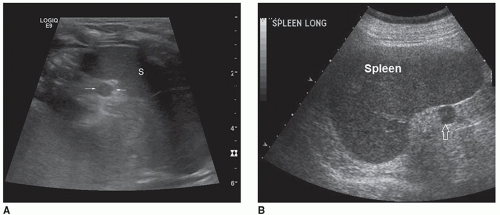
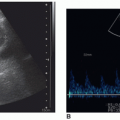
The normal echogenicity of the spleen is comparable with that of the liver and equal to or slightly hyperechoic to the kidney when the tissues are evaluated at the same distance from the transducer.6 Splenic parenchyma is homogeneous with low to mid-level echoes, which are usually disrupted only by the arteries and veins in the area of the hilus (Fig. 10-6). Within the splenic hilum, the splenic artery, and its bifurcations, along with convergence of the splenic veins are visualized. Differentiation between these vessels is difficult without the evaluation of their Doppler signals6,9 (Fig. 10-7).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree